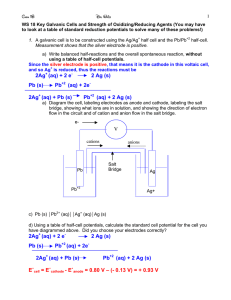
U4 S2 L2 E cell
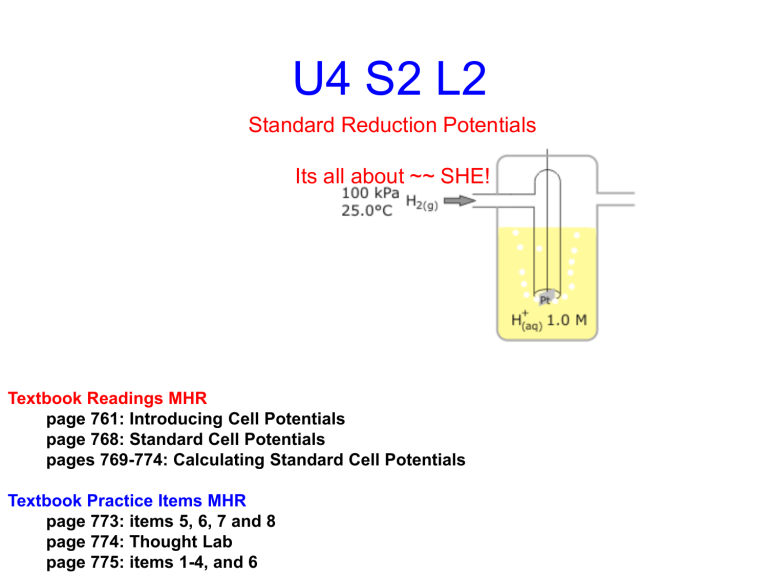
U4 S2 L2
Standard Reduction Potentials
Its all about ~~ SHE!
Textbook Readings MHR page 761: Introducing Cell Potentials page 768: Standard Cell Potentials pages 769-774: Calculating Standard Cell Potentials
Textbook Practice Items MHR page 773: items 5, 6, 7 and 8 page 774: Thought Lab page 775: items 1-4, and 6
Upon completion of this lesson, you should be able to:
• define half-cell voltage, standard half-cell, cell voltage, E°
• use a standard reduction potential table to predict cell voltage and to predict if the redox reaction is spontaneous (occurs as it is written)
• develop a table of redox half-reactions from experimental results
• Electrons spontaneously flow from a position of higher potential energy
(anode) to a position of lower potential energy (cathode).
• The moving electrons can do work; light bulbs, motors….
• Electric potential of a cell:
E p 761
– The difference between the potential energy of the anode and the cathode .
• Measured in volts (V)
• Commonly called – cell voltage or cell potential
• A cell potential of 0 V (zero) means the cell has no electric potential.
– If the electrodes are the same, then there is no difference in there potential and will not generate any electric potential (same height)
– Electrodes must be different.
Standard cell potentials p 768
• The standard half cell table of reduction potentials was created by comparing all half cell reactions with the standard hydrogen electrode (SHE)
• The SHE has H
1.0 mol/l HCl
2(g)
(aq). bubbled over a Pt electrode in
This half cell has been given a E 0 =
0.00 V.
Standard reduction potentials of half cells
Table 19.1 p 768 or Table E.14 p 848
These are standard values which means:
1. all elements in the table are in their standard states at 25 o C and 101.3 kPa .
2. And all ions have a standard molar concentration of 1.0 mol/L .
Keep in mind that all half cells are written as reduction.
– For oxidation half reactions we need to reverse the equation and change the sign!
• Strongest reducing agent is lowest on the right on the table.
• Strongest oxidizing agent is highest on the left
• Calculate the E o for the following cell:
Zn
(s)
| Zn 2+
(1.0mol/L)
|| Cu 2+
(1.0mol/L)
| Cu
(s)
– Formula method:
E o cell
E o cathode
o
E anode
– Half cell method:
• Calculate the E 0 for this reaction:
Which half cell will be the anode?
Zn | Zn
2+
(1 mol/L)
|| H
+
(1 mol/L)
|Pt,H
2
Spontaneity – will electrons flow!
p 770
• If a cell is to produce an electric current the E 0 cell must be positive .
– That is, the cathode must have a higher reduction potential than the anode .
•
Ie: On the table, the cathode must be higher on the table than the anode .
Calculate the cell potential for each: Indicate if a current can be produced.
Cd | Cd 2+ || Cu 2+ | Cu
I
2
| 2 I || 2 Cl | Cl
2
Reduction table trends:
• Which will react spontaneously with Br
2 but not with I
2
?
– Ie: On the table, the cathode must be higher on the table than the anode .
a) Cr 2+ b) F c) Fe 2+ d) Mn 2+
• P 775 #2c