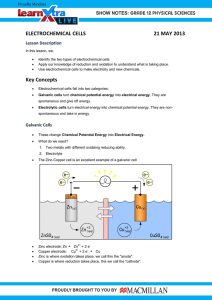
Electrochemistry Questions: Chang Chemistry 10th Ed.
advertisement
Chemistry Raymond Chang 10th edition Chapter 19 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Question 3 The purpose of a salt bridge in an electrochemical cell is 25% 25% A) to give electrons a path to flow between the cathode and anode. B) to allow the reactants to mix so that reaction can occur. C) to maintain a balance of charge between the two half cells and thus allow a complete circuit. D) all of the above. 1 2 25% 3 25% 4 Question 5 The reduction of one mole of copper(II) ions to copper metal requires the transfer of one Faraday (about 96,500 Coulombs) of charge. A) True B) False 50% 50% 1 2 Question 7 The electrochemical potential of a cell depends on which of the following? 25% A) The standard reduction potential for the half-reaction that occurs at the anode. B) The standard reduction potential for the half-reaction that occurs at the cathode. C) The concentrations of species in the cell. D) All of the above. 1 25% 25% 2 3 25% 4 Question 10 Increasing the concentration of products in an electrochemical reaction will decrease the value of the electrochemical potential for the reaction. A) True B) False 50% 50% 1 2 Question 14 In a galvanic cell 33% 33% 33% A) oxidation takes place at the anode. B) oxidation takes place at the cathode. C) none of the above. 1 2 3 Question 18 It is possible to obtain zinc metal by placing a strip of copper in an aqueous solution of ZnSO4. A) True B) False 50% 50% 1 2