unit_3_part_2_prezi_pp[1]
advertisement
![unit_3_part_2_prezi_pp[1]](http://s3.studylib.net/store/data/009491974_1-00bf7ba85f40f2a9d20b42cbbebf0ab5-768x994.png)
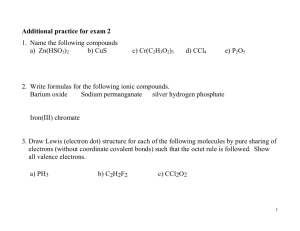
Unit 3: Molecules and Compounds Chemical Formula How to determine the charge of an ion: If an atom gains electrons it will become negatively charged (an anion) because it is gaining negative charges. If an atom loses electrons it is losing negative charges so it will be positive (a cation). For each of the atoms listed below, determine how many valence electrons they have, how many valence electrons they would need to gain or lose to fill their valence shell, and what the charge would be on the ion with a full valence shell. Element Sulfur Hydrogen Sodium Oxygen # of Valence Electrons # of e- to gain or lose Charge How to Determine Chemical Formula for Ionic Compounds: In order to determine the chemical formula for a compound we need to look at the charges that the atoms would have with a full valence shell. For the transition metals we will always be given its charge. # of Atoms: First we need to be able to read how many atoms are in a molecule: ◦ O= ◦ O2= ◦ Na2O= ◦ 3Na2O= ◦ Mg(OH)2= ◦ (NH4)2SO4= Chemical Formula for Binary Compounds: To figure out how many of each atom goes into a molecule we use the criss-cross method: Sr and Cl Look on the common ion sheet to find the charges: Drop the Charge (leave the number): Criss Cross the numbers: Ones do not need to be written: Ba and O Cu and F Chemical Formula for Ionic Compounds that Contain Polyatomic Ions For polyatomic ions, we look on the common ion sheet to find their charge. We use this charge in our criss cross method. Ammonium NH4+1 and Ferrocyanide Fe(CN)6 4Polyatomic ions need to have brackets around them (if there is more than one) Magnesium and Carbonate Cobalt (III) and Sulfate (See Determining Number of Atoms and Criss Cross Assign Pg. 37) How to determine the Lewis dot structure for ionic compounds Example: Determine the lewis dot structure for the ionic compound that will form between calcium and chlorine. Determine the number of atoms of each element there will be (Criss Cross). Draw out the lewis dot structure for each of the atoms. Circle the electrons that are going to leave and create the cation and draw arrows to the anion they are going to. Draw out the lewis dot for each of the ions created and put them in brackets with the charge in the upper right corner outside of the brackets. Example: Aluminum and Oxygen (See Ionic Bonding Lewis Dot Assign Pg. 38)