Lewis Dots and Ions Worksheet
advertisement
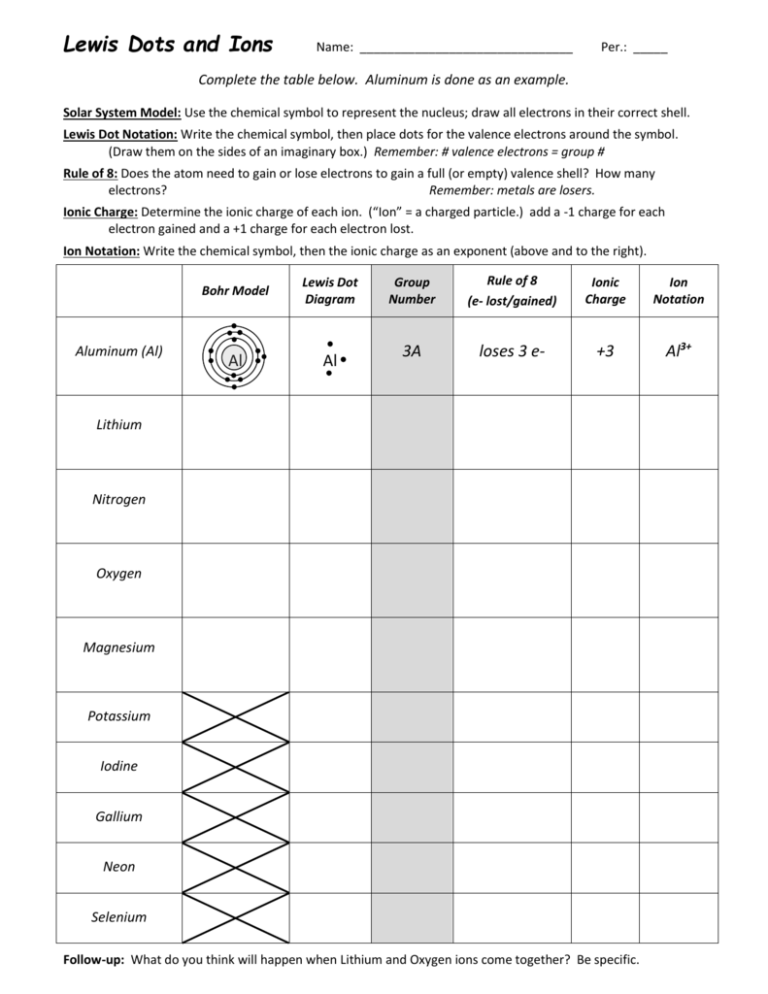
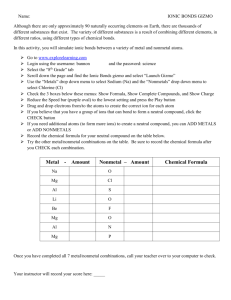
Lewis Dots and Ions Name: _______________________________ Per.: _____ Complete the table below. Aluminum is done as an example. Solar System Model: Use the chemical symbol to represent the nucleus; draw all electrons in their correct shell. Lewis Dot Notation: Write the chemical symbol, then place dots for the valence electrons around the symbol. (Draw them on the sides of an imaginary box.) Remember: # valence electrons = group # Rule of 8: Does the atom need to gain or lose electrons to gain a full (or empty) valence shell? How many electrons? Remember: metals are losers. Ionic Charge: Determine the ionic charge of each ion. (“Ion” = a charged particle.) add a -1 charge for each electron gained and a +1 charge for each electron lost. Ion Notation: Write the chemical symbol, then the ionic charge as an exponent (above and to the right). Aluminum (Al) Bohr Model Lewis Dot Diagram Al Al Group Number Rule of 8 (e- lost/gained) Ionic Charge Ion Notation 3A loses 3 e- +3 Al3+ Lithium Nitrogen Oxygen Magnesium Potassium Iodine Gallium Neon Selenium Follow-up: What do you think will happen when Lithium and Oxygen ions come together? Be specific.