Review for Test #1 Properties of Matter

SCH 4C Review for Test #1 Properties of Matter
Using your class notes and lab results, you should review and understand the following topics:
Difference between physical properties and chemical properties. Identify some.
Bohr model of the atom-
-what are line spectra? How are they created? (describe what electrons are doing)
Atomic structure- atomic #, mass #, protons, electrons, neutrons
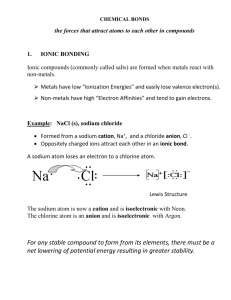
How elements form ions-
-what are valence electrons?
-how do you decide whether a neutral atom will gain or lose electrons? (octet rule)
Draw Lewis dot symbols for atoms and their ions (first 20 only)
Properties of ionic vs. covalent substances (see results of lab work in your duo-tang)
Electronegativity and types of bonds (is
EN more or less than 1.7?)
Ionic bonding. When does it happen? Illustrate it using Lewis dot diagrams.
Covalent bonding. When does it happen? Illustrate it using Lewis dot diagrams.
Polar molecules. How can you tell if a molecule is polar? How does molecular polarity affect observed properties such as melting point?
Review Questions
1. Electrons can be found in either the ground state or the excited state. a) Explain how an electron is promoted from the ground state to the excited state. b) In which state does the electron possess more energy? c) What happens when an electron returns to the g round state?
2. Why may solutions that contain an ionic solute conduct electricity?
3. Draw a Lewis symbol for each of the following atoms and ions: a) sodium ion d) phosphorus atom b) calcium ion e) neon atom c) oxygen atom f) chloride ion
4. Identify which ions in question 3 are negatively charged and which ions are positively charged.
Indicate how many electrons each atom has gained or lost when forming the ion.
5. Which of the following pairs of atoms would you expect to form ionic compounds? Give reasons for your answer. a) sodium and fluorine b) carbon and hydrogen c) magnesium and chlorine
6. Describe one similarity and one difference between a covalent bond and an ionic bond.
7. Draw a lewis structure for the compound that consists of each of the following pairs of atoms. a) hydrogen and nitrogen b) oxygen and oxygen c) hydrogen and oxygen d) nitrogen and nitrogen
8. Why is water a polar molecule?
9. Why is carbon tetrachloride, CCl
4
, a nonpolar molecule even though the C-Cl bond is polar?
10. Molecular solids and ionic solids differ in many ways. Given the information below, determine whether each solid is ionic or molecular.
Solid Melting Point ( o C) Boiling Point ( o C)
A 776 1500
Conductivity in aqueous solutions
Good
B 76 196 none



![unit_3_part_2_prezi_pp[1]](http://s3.studylib.net/store/data/009491974_1-00bf7ba85f40f2a9d20b42cbbebf0ab5-300x300.png)