KI, LiF, K2Se, Na2S, MgO, CaO, CaCl2, and Na3N
advertisement

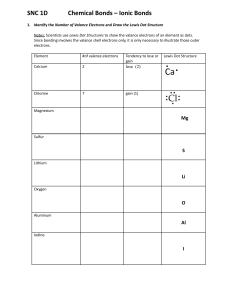
Name___________________________ Date_____________Section_________ Ionic Bonding and Lewis Dot Structures On a sheet of loose-leaf paper, complete #1 through #4 using the examples as a guide for the following ionic compounds: KI, LiF, K2Se, Na2S, MgO, CaO, CaCl2, and Na3N 1. Name the compound. 2. Identify the cation, its charge and electrons lost. Identify the anion, its charge and electrons gained. 3. You must also draw the Lewis Dot Diagram for each ion. 4. Draw the Lewis Dot Structure for the ionic compound making sure that the compound contains the proper number of each ion and is electrically neutral. You will have to follow the examples exactly and provide all the information to receive full credit.