HW Chapter 1
advertisement

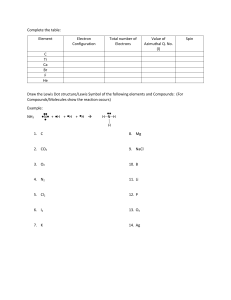
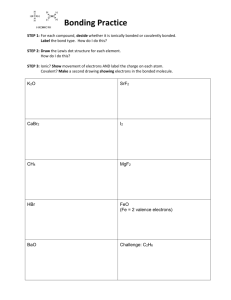
CHM 201 HW chapter 1-part 1 Name Due Tuesday, 1/10/12 *Show your work clearly and staple your papers OR you will get ZERO 1. Draw an orbital electron configuration for the following atom then i. Determine the core electrons ii. Determine the valence electrons a. Mg b. As c. Cd 2. Calculate the formal charges on all of the atoms, except hydrogens, in these compounds: a. b. 3. Using δ+ and δ- to show the direction of polarity of indicated bond in each of the following compounds: a. C – Br b. C—S c. N–H 4. Show a Lewis structure for urea, CH4N2O. Both N’s and the O are bonded to the C. The H’s are bonded to the N’s. None of the atoms has formal charge. 5. Show a Lewis structure for cyanate anion (OCN-). Which atom has the negative formal charge in your structure? Show its resonance structure