Intro to Bonding & Lewis Dot Models Chemistry Notes
advertisement

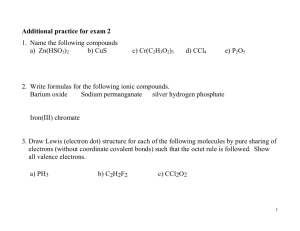
10/14 Notes on Intro to Bonding and Lewis Dot Models Bellringer: What are ions? What are the 2 types of ions? How do you think that chemical compounds are formed? In nature, only the noble gases exist as monoatomic, or single isolated atoms Many elements exist as molecules. Molecules are made up of 2 or more atoms of the same element that act as a unit. Example: Diatomic molecules: each molecule has 2 atoms of the same element. There are 7 elements that exist as diatomic molecules in nature: H2, N2, O2, F2, Cl2, Br2, I2 Ozone: Triatomic molecule of oxygen – 3 oxygens combined in each molecule What about all the other elements besides the noble gases and diatomics? They form compounds: A combination of 2 or more different elements There are 2 types of compounds: Molecular and ionic Molecular: Combinations of nonmetals. Example CO, H2O, CH4, CO2 Ionic: Compounds made up of combinations of ions; Combinations of at least one metal and at least one nonmetal. Examples: NaCl, CaCO3 NaCl : Na tends to lose 1 electron forms +1 ion, Cl tends to gain 1 electron, forms -1 ion Opposite charges attract, this causes the two ions to become attracted and form an ionic bond Remember: Valence electrons are the electrons that interact in a chemical reaction. The same is true for bonding. New way to represent valence electrons: Lewis dot structures: Up to 8 dots are placed around the symbol of the element. The 8 dots represent the number of valence electrons. Example: Na. Put one dot by the Na since Na has 1 valence electron ::Cl:. 7 dots since Cl has 7 valence electrons ::Xe:: 8 valence electrons What about ions? ::Cl:. + e- ::Cl-:: Na – e- Na+ Now its your turn: Draw the lewis dot structures
![unit_3_part_2_prezi_pp[1]](http://s3.studylib.net/store/data/009491974_1-00bf7ba85f40f2a9d20b42cbbebf0ab5-300x300.png)