S2 Final Exam Practice Problems
advertisement
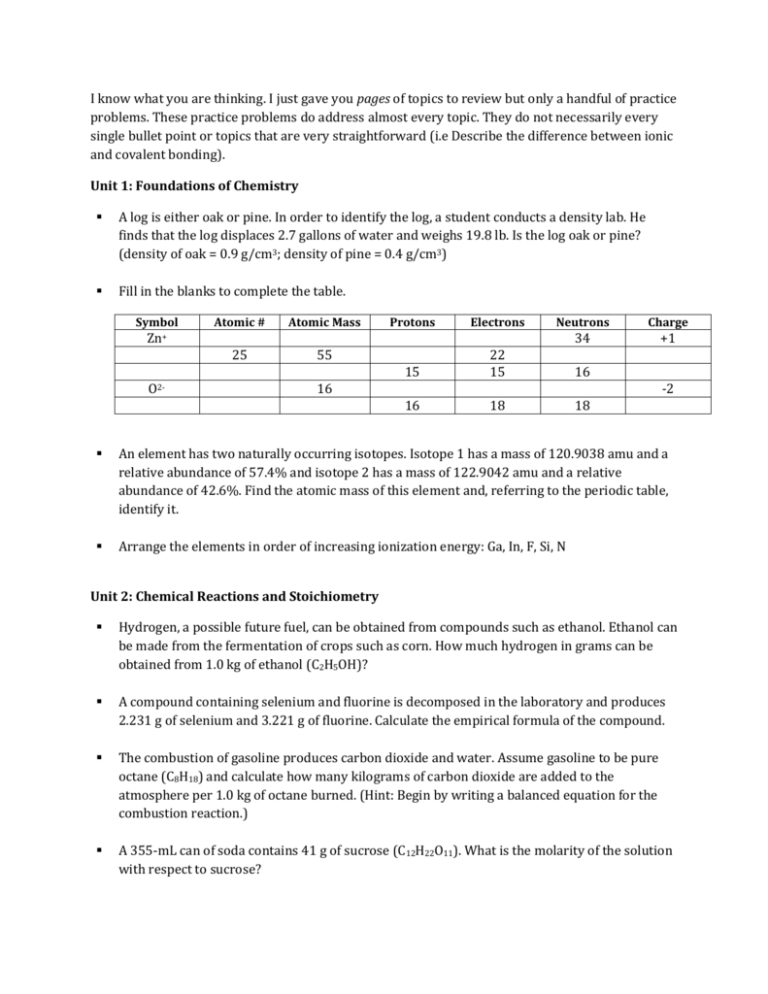
I know what you are thinking. I just gave you pages of topics to review but only a handful of practice problems. These practice problems do address almost every topic. They do not necessarily every single bullet point or topics that are very straightforward (i.e Describe the difference between ionic and covalent bonding). Unit 1: Foundations of Chemistry A log is either oak or pine. In order to identify the log, a student conducts a density lab. He finds that the log displaces 2.7 gallons of water and weighs 19.8 lb. Is the log oak or pine? (density of oak = 0.9 g/cm3; density of pine = 0.4 g/cm3) Fill in the blanks to complete the table. Symbol Atomic # Atomic Mass Protons Electrons Neutrons Charge 34 +1 Zn+ 25 O2- 55 15 22 15 16 16 18 18 16 -2 An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4% and isotope 2 has a mass of 122.9042 amu and a relative abundance of 42.6%. Find the atomic mass of this element and, referring to the periodic table, identify it. Arrange the elements in order of increasing ionization energy: Ga, In, F, Si, N Unit 2: Chemical Reactions and Stoichiometry Hydrogen, a possible future fuel, can be obtained from compounds such as ethanol. Ethanol can be made from the fermentation of crops such as corn. How much hydrogen in grams can be obtained from 1.0 kg of ethanol (C2H5OH)? A compound containing selenium and fluorine is decomposed in the laboratory and produces 2.231 g of selenium and 3.221 g of fluorine. Calculate the empirical formula of the compound. The combustion of gasoline produces carbon dioxide and water. Assume gasoline to be pure octane (C8H18) and calculate how many kilograms of carbon dioxide are added to the atmosphere per 1.0 kg of octane burned. (Hint: Begin by writing a balanced equation for the combustion reaction.) A 355-mL can of soda contains 41 g of sucrose (C12H22O11). What is the molarity of the solution with respect to sucrose? Aspirin can be made in the laboratory by reacting acetic anhydride (C4H6O3) with salicylic acid (C7H6O3) to form aspirin (C9H8O4) and acetic acid (C2H4O2). The balanced equation is: C4H6O3 + C7H6O3 C9H8O4 + C2H4O2 In a laboratory synthesis, a student begins with 5.00 mL of acetic anyhydride (density = 1.08 g/mL) and 2.08 g of salicylic acid. Once the reaction is complete, the student collects 2.01 g of aspirin. Determine the limiting reactant, theoretical yield of aspirin, and percent yield of aspirin. Unit 3: Thermochemistry Two solid objects, A and B, are placed in boiling water and allowed to come to temperature there. Each is then lifted out and placed in separate beakers containing 1000 g water at 10.0°C. Object A increases the water temperature by 3.50°C; B increases the water temperature by 2.60°C. Which object has a larger specific heat? Provide calculations to support your answer. Consider the following hypothetical reactions: AB ΔH = + 30 kJ BC ΔH = + 60 kJ a.) Use Hess’ law to calculate the enthalpy change for the reaction A C. b.) Construct an enthalpy diagram for substances A, B, and C. Potassium hydrogen phthalate (KHC8H4O4, molar mass 204.23 g/mol), commonly known as KHP, is an acid that neutralizes NaOH according to the following reaction: KHC8H4O4 (aq) + NaOH (aq) H2O (l) + KNaC8H4O4 (aq) In a separate experiment done to determine the enthalpy change associated with this neutralization reaction, a 50.0 mL aqueous solution of KHP is placed in a coffee-cup calorimeter and 50.0 mL of 0.213 M NaOH are added to the calorimeter. The initial temperature of both solutions is 22.9°C and the temperature increases to 24.2°C. Calculate the ∆Hrxn in kJ/mol NaOH for this reaction assuming the specific heat of the solutions is 4.18 J/g·C. Unit 4: Electronic Structure and Chemical Bonding In terms of atomic structure, explain why the ionization energy of selenium is less than that of bromine but greater than that of calcium. When an electron makes a transition from the n=3 to the n=2 hydrogen atom Bohr orbit, the energy difference between these two orbits, 3.0 x 10-19 J, is emitted as a photon of light. Calculate the wavelength, in nm, of the photon of light. Explain what is wrong with each electron configuration and write the correct ground state (or lowest energy) configuration based on the number of electrons. (3 points each 5 points total) a. 1s32s32p9 b. 1s22s22p62d4 c. 1s21p5 d. 1s22s22p83s23p1 e. 1s22p63s2 Unit 5: States of Matter How many grams of hydrogen are collected in a reaction where 1.78 L of hydrogen gas is collected over water at a temperature of 40°C and a total pressure of 748 torr. A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mmHg. The partial pressures of helium and nitrogen are 234 mmHg and 197 mmHg respectively. What is the partial pressure of oxygen in the mixture? Which gas sample depicted, all at the same temperature, will have the greatest pressure? Explain. Sample A Sample B Sample C