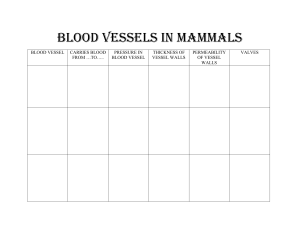
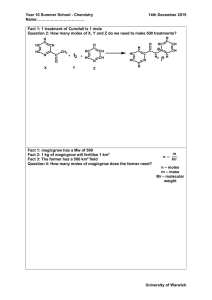
Collecting Gases Over Water/Dalton`s Law Example Problem
advertisement

Collecting Gases Over Water/Dalton’s Law Example Problem A 300. mL sample of hydrogen gas was collected over water at 21 oC at an atmospheric pressure of 748 Torr. (Assume the collection vessel height was adjusted so that the level of water inside and outside the vessel are equal). a. How many moles of hydrogen gas were present? b. What is the mole fraction of H2 in the moist gas mixture? c. What is the mass of the dry H2? d. What volume would the H2 occupy if the gas was transferred to conditions of STP?