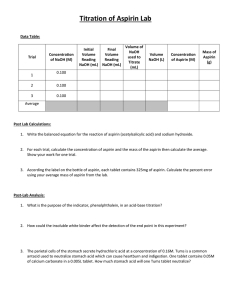
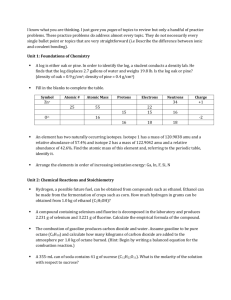
Read this entire section before you begin working on the lab.
advertisement

Hints for writing your answers to the pH lab. Read this entire section before you begin working on the lab. Discuss the answers with each other and make sure your answers are complete and clearly explained. This is an opportunity to discuss the answers with other students and write down important points that you will be including in your final answers. If you use this time properly you will have a much easier time writing out your final answers at home. Today, you should be writing in the lab that we used in class. This lab will not be handed in so it doesn’t have to be neat. You will eventually type your answers into the answer packet that is printed on line. As I mentioned on the web site, your final answers on the lab you hand in must be in complete sentences with correct spelling and proper grammar. At home, after you type up your answers, go back and reread it to check that it makes sense. You definitely need to check and edit your work before you hand it in. You will often find that what you originally wrote doesn’t make sense - it wasn’t what you meant to say. This is why you should always edit your work before you hand it in. What is a ‘good answer’? Look at page 3, #5 . After you describe the color change you must discuss what causes it to change color. ex- After I added the NaOH the color of the solution changed from clear to pink. This is because............... Your answer should include the following terms and items: base or basic, dissociate, the indicator phenolphthalein, Na+, OH-, H3O+ . Make sure to look at the Beaker diagrams we drew in class. Look at what happened when we added NaOH and HCl. This will help you construct a good answer. For the chart on Page 1, after pH, just put a check in the appropriate box for H+ or OH-. Then just put the word acidic, basic or neutral. You should check the pH values with the students in your group. Make sure you have a basic pH for Maalox, and that the buffered aspirin is more basic than the regular aspirin. Regular aspirin should be acidic. Page 8 should have information on pH and acid rain. It seems that this page is blank on your lab. On the back of this page is the new page 8 with the article on acid rain. When you get to the question on buffered aspirin vs. regular aspirin, you can ask your parents to help you. You should start typing up the answers soon because the entire lab will be due on _______________ for your class.