Chapter 16 Notes, part II
advertisement

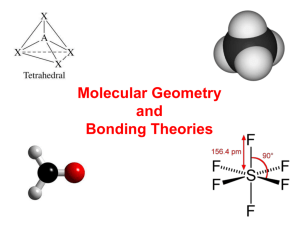
Chapter 16 Notes, part II More on Covalent Bonding Drawing Line Diagrams • A line diagram replaces the circled shared pair of electrons with a line. • Lines must be straight and short; double bonds should look like an equal sign. • Draw line diagrams for: H 2O CO2 HCN Resonance • If you draw the covalent bonding diagram of some compounds—nitrite for example—there is not only one way to draw it. • There are two valid ways to show the structure of nitrite. The real structure is an average of the two, and this is called a resonance structure. It is shown by: Resonance, cont. • Earlier chemists thought the compound just quickly flipped back and forth between the two structures (or resonated). • This is proven to be untrue now using bond lengths. Coordinate Covalent Bonding • A coordinate covalent bond occurs when one atom gives both electrons to a shared pair between them. • In a line diagram, instead of a straight line showing a shared pair, the coordinate covalent bond is shown as an arrow. Sigma and Pi Bonds • How do covalent bonds form between two elements? • By the combining of their p orbitals. Sigma and Pi Bonds • The first bond between two atoms is a sigma bond (s) and it forms because of end-to-end overlap of p orbitals. • Any other bonds between the same atoms would be a pi bond (p) and they form because of side-to-side overlap of p orbitals. Sigma and Pi Bonds • Single bond = 1 sigma bond • Double bond = 1 sigma + 1 pi bond • Triple bond = 1 sigma + 2 pi bonds Sigma and Pi Bonds • How many sigma and pi bonds do the following compounds have? NH3 CH2O SiO2 H2O HCN CS2 So, then how… • If bonding occurs only with p orbitals, how does carbon make 4 bonds? • By forming hybrid orbitals! Hybrid Orbitals • The s-orbital, which is not normally used for bonding, combines with the p-orbitals to make 4 equal energy bonding orbitals. Hybrid Orbitals • Some bigger elements will also form hybrid orbitals using their d-sublevel’s orbitals. • This allows for compounds that wouldn’t normally be able to form—compounds that break the octet rule. Exceptions to the Octet Rule • PCl5 • SF6 • BF3