igcse fa rev ch3
advertisement
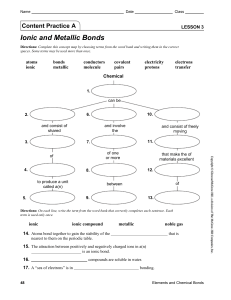
I IGCSE REVISION Chapter 3 The following key words and explanations must be memorized. You should be able to explain some of them with examples too. The words in bold need a definition. Look up the definition in the glossary of your revision guide or in your textbook. These must be memorized word for word. Section/ Detail Chapter A.3 Covalent Bond Be able to draw the covalent bonds formed between the following molecules Hydrogen H2 Hydrogen Chloride HCl Chlorine Cl2 Water H2O Ammonia NH3 Oxygen O2 Carbon Dioxide CO2 Nitrogen N2 Be able to draw the dot cross diagram for each of the above Be able to represent the bonds with lines for single, double and triple bonds for each of the above Ionic Bonding Ions Cations Anions Explain how ionic bonds form and why. What is the importance of noble gas structure? complete learned Be able to draw full ionic bond diagrams dot cross diagrams electronic structure diagrams for any ionic compound formed between a metal and a non metal eg NaCl, MgO, LiO, KN, CaCl2, Al2O3 Metallic Bonding Recap the properties of metals Describe their structure Link their structure to their properties Ie: high melting point Density Conduction of electricity Be able to explain why metals with more electrons in their outermost shell have a stronger metallic bond and therefore higher melting point see me if you need further explanation