electrochemistry 2
advertisement

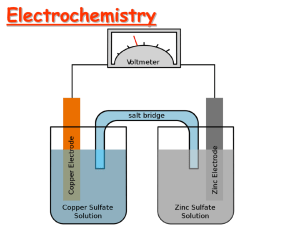
Standard Reference Electrode Standard Hydrogen Electrode (SHE) SHE: • Assigned 0.000 V • Can be anode or cathode • Pt does not take part in reaction • Difficult to operate Standard Conditions: 1 atm for gases, 1.0M for solutions, 25 oC for all (298 K) Alternative reference electrodes • Ag/AgCl electrode AgCl(s) + e- Cl- + Ag(s) Ecell = +0.22 V vs. SHE • Calomel electrode Hg2Cl2(s) + 2e- 2Cl- + 2Hg(l) Ecell = + 0.24 V vs.SHE Standard Reduction Potentials in Aqueous Solution at 25° C How can we determine which substance is being oxidized and which is being reduced? …..The MORE POSITIVE reduction potential gets to be reduced. Reading the reduction potential chart Elements that have the most positive reduction potentials are easily reduced. Elements that have the least positive reduction potentials are easily oxidized. The table can also be used to tell the strength of various oxidizing and reducing agents. It can also be used as an activity series. Metals having less positive reduction potentials are more active and will replace metals with more positive potentials. Zn 1 hour CuSO4 Calculating Standard Cell Potential E o Cell E o Cathode E o Anode E o Re d E o Ox IMPORTANT: Write both equations AS IS from the table in the reduction form with their voltages. Example: Calculate E0 for the cell shown in the Figure below: Cu2+ + 2e- Cu(s) Eo = 0.337 V Zn2+ + 2e- Zn(s) Eo = -0.763 V e- V e- o o o o o ECell ECathode E Anode ERe E d Ox Eo = (0.337) – (-0.763) = +1.10 V Zn Zn2 + Cu NO3- Cu2+ NO3 - Remember: -Cu should be the cathode (it has higher Eo). - Oxidation occurs at the anode (may show mass decrease). - Reduction occurs at the cathode (may show mass increase). - In terms of electrode charge Electrolytic cell Anode (+) Cathode (-) Galvanic cell Anode (-) Cathode (+) - (°) means standard conditions: 1atm, 1M, 25C. - Negative Eo implies non-spontaneous - Positive Eo implies spontaneous (would be a good battery!). Example: Consider a galvanic cell based on the reaction: Ag+(aq) + Sn(s) → Ag(s) + Sn2+(aq) Give the balanced cell reaction and calculate E° for the cell. Ag+ + e- Ag(s) Sn2+ + 2e- Sn(s) Eo = 0.80 V o o o ECell ECathode E Anode Eo = -0.14 V o o EAg ESn 2Ag+ + Sn 2Ag(s) + Sn2+ Eo = 0.80 – (-0.14) = 0.94 V Example: Will the following mixture react spontaneously at standard state? Zn2+(aq) + Fe2+(aq) ??? Given: (a) Zn2+ + 2e- (b) Fe3+ + e- Zn Fe2+ (a) -2(b): Zn2+ + Fe2+ Zn(s) Eo= -0.76 V Eo = 0.77 V + 2Fe3+ E0 for the overall reaction = -0.76 – (0.77) = -1.53 V The forward reaction is not spontaneous. Example: Using the table of standard reduction potentials, predict whether 1 M HNO3 will dissolve gold metal to form a 1 M Au3+ solution. …… ….. No! Au3+ + 3e- Au(s) Eo = 1.50 V NO3- +4H3O+ + 3e- NO(g) + 6H2O(l) Eo = 0.96 V To dissolve Au, it should be as Au3+ NO3- +4H3O+ + Au Au3+ + NO(g) + 6H2O(l) o o o ECell ECathode E Anode 0.96 1.50 0.54V Eo = ?? V Dependence of Cell Potential on Concentration Voltaic cells at non-standard conditions -- LeChatlier’s principle can be applied. An increase in the concentration of a reactant will favor the forward reaction and the cell potential will increase. The converse is also true! Example: For the cell reaction: 2Al + 3Mn2+ → 2Al3+ + 3Mn E°cell = ?? Predict whether Ecell is larger or smaller than E°cell for the following cases: a. [Al3+ ] = 2.0 M, [Mn2+ ] = 1.0 M A: Ecell < E°cell b. [Al3+ ] = 1.0 M, [Mn2+] = 3.0 M B: Ecell > E°cell When cell is not at standard conditions, use Nernst Equation • In a chemical reaction such as: aA + bB cC + dD c C a A d D b B a .a o G G 2.3RT log G 2.3RT log Q a .a o Substitute G nFE G o nFE o Where: Go : Free energy change when all the reactants and products are in their standard states (unit activity). R : is the gas constant. T : is the temperature in the absolute temperature Q : Reaction Quotient aCc .aDd G G 2.3RT log a b a A .aB o c d [ C ] [ D ] nFE nFE o 2.3RT log [ A]a [ B]b c d 2 . 3 RT [ C ] [ D ] E Eo log nF [ A]a [ B]b Where concentrations are substituted for activities At 298 K the equation becomes K c d 0 . 0591 [ C ] [ D ] E Eo log …… Nernst Equation n [ A]a [ B]b At Equilibrium, G = 0, E = 0. Hence 0.0591 0E log K n o 0.0591 E log K n o Concentration Cells We can construct a cell where both compartments contain the same components BUT at different concentrations. In the picture, Silver will be deposited on the right electrode, thus lowering the concentration of Ag+ in the right compartment. In the left compartment the silver electrode dissolves [producing Ag+ ions] to raise the concentration of Ag+ in solution. Example: Using the table of standard reduction potentials, calculate ∆G° for the reaction: • Is this reaction spontaneous? Cu2+ + Fe → Cu + Fe2+ ………. Yes! Example : Determine Eocell and Ecell based on the following halfreactions: VO2+ + 2H+ + e- → VO2+ + H2O Zn2+ + 2e- → Zn E° = 1.00 V E° = -0.76V Where: 25°C, [VO2+] = 2.0 M, [H+] = 0.50 M, [VO2+] = 1.0 x 10-2 M, [Zn2+] = 1.0 x 10-1 M o Cell E E o Cathode E o Anode = 1.00 – (-0.76) = 1.76 V 2VO2+ + 4H+ + Zn → 2VO2+ + 2H2O + Zn2+ 0.0591 [VO 2 ]2 .[ Zn 2 ] EE log n [VO2 ]2 .[ H ]4 o 0.0591 (0.01) 2 .(0.1) 1.76 log 1.89V 2 4 2 (2.0) .(0.5) Example: Calculate Kw, the ion-product constant of water, using the given data. H 2 O + e- H+ + e- 1/2H2 + OH- Eo = -0.83 V Eo = 0.00 V 1/2H2 K w [ H ].[OH ] Should be Anode Therefore, we need this equation: the ion-product H2O H+ + OH- E oCell E ocathode E oAnode (0.83) (0) 0.83V E oCell 0.83 0.0591* log[ H ].[OH ] 0 0.83 0.0591* log Kw Kw = 1 x 10-14 At equilibrium, Ecell = 0, and [H+][OH-] = Kw