Introduction to Electrochemistry
advertisement
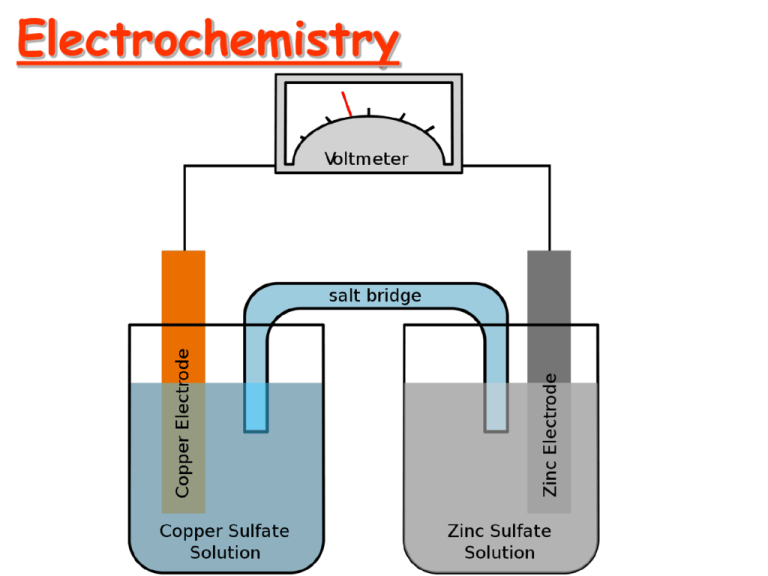
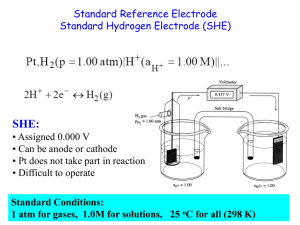
Electrochemistry Electrochemistry Terminology #1 Oxidation – A process in which an element attains a more positive oxidation state Na(s) Na+ + eReduction – A process in which an element attains a more negative oxidation state Cl2 + 2e- 2Cl- Electrochemistry Terminology #2 An old memory device for oxidation and reduction goes like this… LEO says GER Lose Electrons = Oxidation Gain Electrons = Reduction Electrochemistry Terminology #3 Oxidizing agent The substance that is reduced is the oxidizing agent Reducing agent The substance that is oxidized is the reducing agent Electrochemistry Terminology #4 Anode The electrode where oxidation occurs Cathode The electrode where reduction occurs Memory device: Reduction at the Cathode Table of Reduction Potentials Measured against the Standard Hydrogen Electrode Measuring Standard Electrode Potential Potentials are measured against a hydrogen ion reduction reaction, which is arbitrarily assigned a potential of zero volts. Galvanic (Electrochemical) Cells Spontaneous redox processes have: A positive cell potential, E0 A negative free energy change, (-G) Zn - Cu Galvanic Cell From a table of reduction potentials: Zn2+ + 2e- Zn Cu2+ + 2e- Cu E = -0.76V E = +0.34V Zn - Cu Galvanic Cell The less positive, or more negative reduction potential becomes the oxidation… Zn Zn2+ + 2eCu2+ + 2e- Cu E = +0.76V E = +0.34V Zn + Cu2+ Zn2+ + Cu E0 = + 1.10 V Line Notation An abbreviated representation of an electrochemical cell Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) Anode Anode | material solution || Cathode solution | Cathode material Calculating G0 for a Cell G0 = -nFE0 n = moles of electrons in balanced redox equation F = Faraday constant = 96,485 coulombs/mol e- Zn + Cu2+ Zn2+ + Cu E0 = + 1.10 V coulombs Joules G ( 2 mol e )(96 485 )(1.10 ) mol e Coulomb 0 G 212267 Joules 212 kJ 0 The Nernst Equation Standard potentials assume a concentration of 1 M. The Nernst equation allows us to calculate potential when the two cells are not 1.0 M. RT EE ln(Q) nF 0 R = 8.31 J/(molK) T = Temperature in K n = moles of electrons in balanced redox equation F = Faraday constant = 96,485 coulombs/mol e- Nernst Equation Simplified At 25 C (298 K) the Nernst Equation is simplified this way: 0.0591 EE log(Q) n 0 Equilibrium Constants and Cell Potential At equilibrium, forward and reverse reactions occur at equal rates, therefore: 1. The battery is “dead” 2. The cell potential, E, is zero volts Modifying the Nernst Equation (at 25 C): 0.0591 0 volts E log( K ) n 0 Calculating an Equilibrium Constant from a Cell Potential Zn + Cu2+ Zn2+ + Cu E0 = + 1.10 V 0.0591 0 volts 1.10 log( K ) 2 (1.10)(2) log( K ) 0.0591 37.2 log( K ) 10 37.2 K 1.58 x 10 37 ??? Concentration Cell Both sides have the same components but at different concentrations. Step 1: Determine which side undergoes oxidation, and which side undergoes reduction. ??? Anode Concentration Cell Cathode Both sides have the same components but at different concentrations. The 1.0 M Zn2+ must decrease in concentration, and the 0.10 M Zn2+ must increase in concentration Zn2+ (1.0M) + 2e- Zn (reduction) Zn Zn2+ (0.10M) + 2eZn2+ (1.0M) Zn2+ (0.10M) (oxidation) ??? Concentration Cell Anode Cathode Concentration Cell Both sides have the same components but at different concentrations. Step 2: Calculate cell potential using the Nernst Equation (assuming 25 C). Zn2+ (1.0M) Zn2+ (0.10M) 0.0591 EE log(Q) n 0 Nernst Calculations Zn2+ (1.0M) Zn2+ (0.10M) 0.0591 EE log( Q) n 0 E 0.0 Volts 0 n2 (0.10) Q (1.0) 0.0591 0.10 E 0.0 log( ) 0.030 Volts 2 1.0 Electrolytic Processes Electrolytic processes are NOT spontaneous. They have: A negative cell potential, (-E0) A positive free energy change, (+G) Electrolysis of Water In acidic solution Anode rxn: 2 H 2O O2 4 H 4e Cathode rxn: 4 H 2O 4e 2 H 2 4OH 2 H 2O 2 H 2 O2 -1.23 V -0.83 V -2.06 V Electroplating of Silver Anode reaction: Ag Ag+ + eCathode reaction: Ag+ + e- Ag Electroplating requirements: 1. Solution of the plating metal 2. Anode made of the plating metal 3. Cathode with the object to be plated 4. Source of current Solving an Electroplating Problem Q: How many seconds will it take to plate out 5.0 grams of silver from a solution of AgNO3 using a 20.0 Ampere current? Ag+ + e- Ag 5.0 g 1 mol Ag 1 mol e- 96 485 C 1 s 20.0 C 1 mol e 107.87 g 1 mol Ag = 2.2 x 102 s