8.6 - Tying it All Together
advertisement

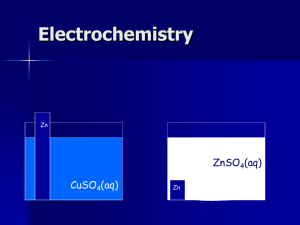
Catalyst You create a Zn/H cell and the potential is 0.45 V at 25 oC when [Zn2+] = 1.0 M and PH2 = 1.0 atm, what is the [H+] What is the pH of the solution in the cathode half – cell for a Zn/H cell where PH2 = 1.0 atm, [Zn2+] = 0.10 M, and the cell emf is 0.542 V. End Heartbeat and Electrochemistry Lecture 8.6 – Concentration Cells, Free Energy and Electrochemistry Today’s Learning Targets LT 8.15 – I can calculate the non-standard electrode potentials and/or concentrations for a concentration cell utilizing the Nernst equation LT 8.16 – I can calculate the free energy of a reaction utilizing the standard reduction potential for the reaction. LT 8.17 – I can calculate the equilibrium constant for a chemical reaction utilizing the standard reduction potential for the reaction. Concentration Cells Cell emf depends on the concentration in each cell also We can construct a cell where both the anode and cathode have the same ion, but there are different concentrations The more dilute solution goes in the anode The more concentrated solution goes in the cathode The driving force is the entropy of mixing Electrochemistry Lab In the electrochemistry lab we studied the following concentration cell: Anode : Ag (s) Ag + (aq,dilute) + e - E o = 0.80 V Cathode : Ag + (aq,concentrated) + e - Ag (s) E o = 0.80 V Therefore, the Eo for this solution would be: o o o E cell E cathode E anode 0.80 0.80 0 V If we add the reactions together we discover that the reaction is the concentrated going to the dilute Ag+: Ag+ (aq, concentrated) Ag+ (aq, dilute) We can solve for E using the Nernst equation and Q is simply: [Agdilute ] Q [Agconcentrated] Class Example A voltaic cell is constructed with two hydrogen electrodes. Electrode 1 has a PH2 1.00 atm and an unknown concentration of H+. Electrode 2 is a standard hydrogen electrode (PH2 =1 atm, [H+] = 1 M). At 298 K, the measured cell potential is 0.211 V and the electrical current is observed to flow from electrode 1 through the external circuit to electrode 2. Calculate [H+] for the solution at electrode 1. What is the pH of the solution? Table Talk A concentration cell is constructed with two Zn (s)-Zn2+ (aq) half-cells. In one half cell [Zn2+] = 1.35 M, and the other [Zn2+] = 3.75 x 10-4 M. (a) Which half cell is the anode? (b) What the emf of this cell? Lab Calculations Complete the lab calculations for questions 1 – 4 on the handout for Part II of the lab last week. Spontaneity and Eo Voltaic cells use a positive Eo in order to produce cell potential Therefore, a Eo must be positive in order for a reaction to be spontaneous This is why we can use standard reduction potentials to predict whether a metal will run in the forward or reverse reaction. Needs to generate a +Eo when reaction is ran. ΔG and Eo Since the emf indicates whether a reaction is spontaneous we can use it to calculate ΔG: G nFE Since n and F are always positive, the sign of E determines the spontaneity of a reaction! Finally we can relate this to equilibrium constants G o nFE G o RT ln K nFE E o o o RT ln K RT ln K nF Class Example Using the standard reduction potentials, calculate the values of ΔG, Eo, and K for the reaction at 298 K: 4 Ag (s) + O2 (g) + 4 H+ 4 Ag+ (aq) + 2 H2O Table Talk Using the standard reduction potentials, calculate the values of ΔG, Eo, and K for the reaction at 298 K: 3 Ni2+ (aq) + 2 Cr(OH)3 (s) + 10 OH- (aq) 3 Ni (s) + 2 CrO42+ + H2O (l) Connecting Concepts! An electrochemical cell is constructed in which one ½ cell contains a buffer of acetic acid (0.100 M) and sodium acetate (0.050 M), a platinum electrode, and H2 bubbled at 1.0 atm. The other half – cell contains a 0.050 M solution of AgNO3 and a silver electrode. Calculate the cell potential for this galvonic cell. Recall that Ka = 1.8 x 10-5 for acetic acid. NOTE – Eo for H+/H2 cell is 0.00 V and Eo for Ag+/Ag (s) cell is 0.960 V. Connecting Concepts! You have the following voltaic cell: Pb (s) | Pb2+ (sat. PbI2) || Pb2+ (aq, 0.150 M) |Pb (s) The Pb2+ cell that contains saturated PbI2 has lead ions in the following equilibrium: PbI2 Pb2+ (aq) + 2 I – (aq) Calculate the Ksp for PbI2 Practice Practice Practice! Closing Time Read 20.5 and 20.6 Homework: Lab Worksheet and calculations due Thursday/Friday Unit 7 Exam Thursday/Friday Stations Review up on the Website!