Electrochemsitry PPT
Electrochemistry
Chapter 19
Electron Transfer Reactions
• Electron transfer reactions are oxidationreduction or redox reactions.
• Results in the generation of an electric current (electricity) or be caused by imposing an electric current.
• Therefore, this field of chemistry is often called ELECTROCHEMISTRY.
Electrochemical processes are oxidation-reduction reactions in which:
• the energy released by a spontaneous reaction is converted to electricity or
• electrical energy is used to cause a nonspontaneous reaction to occur
0 0
2Mg ( s ) + O
2
2+ 2-
( g ) 2MgO ( s )
2Mg 2Mg 2+ + 4e Oxidation half-reaction (lose e )
O
2
+ 4e 2O 2Reduction half-reaction (gain e )
19.1
Terminology for Redox Reactions
• OXIDATION —loss of electron(s) by a species; increase in oxidation number; increase in oxygen.
• REDUCTION —gain of electron(s); decrease in oxidation number; decrease in oxygen; increase in hydrogen.
• OXIDIZING AGENT —electron acceptor; species is reduced. (an agent facilitates something; ex.
Travel agents don’t travel, they facilitate travel)
• REDUCING AGENT —electron donor; species is oxidized.
You can’t have one… without the other!
• Reduction (gaining electrons) can’t happen without an oxidation to provide the electrons.
• You can’t have 2 oxidations or 2 reductions in the same equation. Reduction has to occur at the cost of oxidation
LEO
the lion says
GER
!
o s e i t o n i x d a r o n s t c l e i a n r o n s t c l e i t o n e d u c
GER!
Another way to remember
•OIL RIG
i t o n i x d a s o s e i t o n e d u c s a i n
Review of Oxidation numbers
The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
1. Free elements (uncombined state) have an oxidation number of zero.
Na, Be, K, Pb, H
2
, O
2
, P
4
= 0
2. In monatomic ions, the oxidation number is equal to the charge on the ion.
Li + , Li = +1 ; Fe 3+ , Fe = +3 ; O 2, O = -2
3. The oxidation number of oxygen is usually –2 . In H
2 and O
2
2it is –1 .
O
2
4.4
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is –1 .
5. Group IA metals are +1 , IIA metals are +2 and fluorine is always –1 .
6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion.
HCO
3
-
Oxidation numbers of all the atoms in HCO
3
?
O = -2 H = +1
3x( -2 ) + 1 + ?
= -1
C = +4
4.4
Balancing Redox Equations
The oxidation of Fe 2+ to Fe 3+ by Cr
2
O
7
2in acid solution?
1. Write the unbalanced equation for the reaction in ionic form.
Fe 2+ + Cr
2
O
7
2Fe 3+ + Cr 3+
2. Separate the equation into two half-reactions.
Oxidation:
Reduction:
+6
+2
Fe 2+
Cr
2
O
7
2-
+3
Fe 3+
+3
Cr 3+
3. Balance the atoms other than O and H in each half-reaction.
Cr
2
O
7
22Cr 3+
19.1
Balancing Redox Equations
4. For reactions in acid, add H
2
O to balance O atoms and H + to balance H atoms.
Cr
2
14H + + Cr
2
O
7
2-
O
7
2-
2Cr 3+ + 7H
2
O
2Cr 3+ + 7H
2
O
5. Add electrons to one side of each half-reaction to balance the charges on the half-reaction.
6e + 14H + + Cr
2
Fe 2+
O
7
2-
Fe 3+ + 1e -
2Cr 3+ + 7H
2
O
6. If necessary, equalize the number of electrons in the two halfreactions by multiplying the half-reactions by appropriate coefficients.
6Fe 2+ 6Fe 3+ + 6e -
6e + 14H + + Cr
2
O
7
22Cr 3+ + 7H
2
O
19.1
Balancing Redox Equations
7. Add the two half-reactions together and balance the final equation by inspection. The number of electrons on both sides must cancel. You should also cancel like species.
Oxidation:
Reduction: 6e + 14H + + Cr
2
O
7
2-
14H + + Cr
2
O
7
2+ 6Fe 2+
6Fe 2+ 6Fe 3+ + 6e -
2Cr 3+ + 7H
2
6Fe 3+ + 2Cr 3+ + 7H
2
O
O
8. Verify that the number of atoms and the charges are balanced .
14x1 – 2 + 6x2 = 24 = 6x3 + 2x3
9. For reactions in basic solutions, add OH to both sides of the equation for every H + that appears in the final equation. You should combine H + and OH to make H
2
O.
19.1
CHEMICAL CHANGE --->
ELECTRIC CURRENT
•To obtain a useful current, we separate the oxidizing and reducing agents so that electron transfer occurs thru an external wire.
This is accomplished in a GALVANIC or
VOLTAIC cell.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/galvan5.swf
A group of such cells is called a
battery
.
anode oxidation
-
Galvanic Cells cat hode red uction
+ spontaneous redox reaction
19.2
Galvanic Cells
The difference in electrical potential between the anode and cathode is called:
• cell voltage
• electromotive force (emf)
• cell potential
Cell Diagram
Zn ( s ) + Cu 2+
( aq ) Cu ( s ) + Zn 2+
( aq )
[Cu 2+ ] = 1 M & [Zn 2+ ] = 1 M
Zn ( s ) | Zn 2+ (1 M ) || Cu 2+ (1 M ) | Cu ( s ) anode cathode
19.2
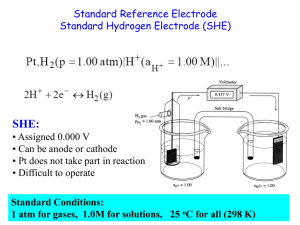
Standard Electrode Potentials
Zn ( s ) | Zn 2+ (1 M ) || H + (1 M ) | H
2
(1 atm) | Pt ( s )
Anode (oxidation): Zn ( s ) Zn 2+ (1 M ) + 2e -
Cathode (reduction): 2e + 2H + (1 M ) H
2
(1 atm )
Zn ( s ) + 2H + (1 M ) Zn 2+ + H
2
(1 atm )
19.3
Standard Electrode Potentials
Standard reduction potential (E 0 ) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm.
Reduction Reaction
2e + 2H + (1 M ) H
2
(1 atm )
E 0 = 0 V
Standard hydrogen electrode (SHE)
19.3
• E 0 is for the reaction as written
• The more positive E 0 the greater the tendency for the substance to be reduced
• The half-cell reactions are reversible
• The sign of E 0 changes when the reaction is reversed
• Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E 0
19.3
Official AP
Reduction Table
Copyright
College Board
Standard Electrode Potentials
Standard emf (E 0 cell
)
E 0 cell
= E 0 cathode
+ E 0 anode
If the reaction is backwards, be sure to flip the sign!
Zn ( s ) | Zn 2+ (1 M ) || H + (1 M ) | H
2
E 0 cell
= E 0
H /H
2
+ E 0
Zn /Zn
+2
(1 atm) | Pt ( s )
Zn 2+ (1 M ) + 2e Zn E 0 = -0.76 V
So E o
Zn/Zn +2
= + 0.76 V
E 0 cell
= 0 + 0.76 V = 0.76 V
19.3
Standard Electrode Potentials
E 0 cell
= 0.34 V
E 0 cell
= E 0 cathode
+ E 0 anode
E 0 cell
= E 0 + E
0
H /H+
2
0.34 = E 0
Cu /Cu
+ - 0
E 0
Cu /Cu
= 0.34 V
Pt ( s ) | H
2
(1 atm ) | H + (1 M ) || Cu 2+ (1 M ) | Cu ( s )
Anode (oxidation): H
2
(1 atm ) 2H + (1 M ) + 2e -
Cathode (reduction): 2e + Cu 2+ (1 M ) Cu (s)
H
2
(1 atm ) + Cu 2+ (1 M ) Cu ( s ) + 2H + (1 M )
19.3
What is the standard emf of an electrochemical cell made of a Cd electrode in a 1.0 M Cd(NO
3
)
2 electrode in a 1.0 M Cr(NO
3
)
3 solution?
solution and a Cr
Cd
Cr
2+
3+
(
( aq aq )
) + 2e
+ 3e -
-
Anode (oxidation):
Cd ( s ) E 0 = -0.40 V Cd is the stronger oxidizer
Cd will oxidize Cr
Cr ( s ) E 0 = -0.74 V
Cr (s) Cr 3+ (1 M ) + 3e x 2
Cathode (reduction): 2e + Cd 2+ (1 M ) Cd (s) x 3
2Cr ( s ) + 3Cd 2+ (1 M ) 3Cd ( s ) + 2Cr 3+ (1 M )
E 0 cell
= E 0 cathode
+ E 0 anode
E 0 cell
= -0.40 + (+0.74)
E 0 cell
= 0.34 V
19.3
Spontaneity of Redox Reactions
D
G = nFE cell n = number of moles of electrons in reaction
D
G 0 = nFE 0 cell
F = 96,500
J
V • mol
= 96,500 C/mol
D
G 0 = RT ln K = nFE 0 cell
E 0 cell
=
RT nF ln K =
(8.314 J/K • mol)(298 K) ln K n (96,500 J/V • mol)
E 0 cell
=
0.0257 V n ln K
E 0 cell
=
0.0592 V n log K
19.4
Spontaneity of Redox Reactions
19.4
What is the equilibrium constant for the following reaction at 25 0 C? Fe 2+
( aq ) + 2Ag ( s ) Fe ( s ) + 2Ag +
( aq )
E 0 cell
=
0.0257 V n ln K
Oxidation: 2Ag 2Ag + + 2e -
Reduction: 2e + Fe 2+ Fe n = 2
E 0 = E 0
Fe /Fe
+ E 0
Ag /Ag
+
E 0 = -0.44 + -0.80
E 0 = -1.24 V
K = exp
E 0 cell x n
0.0257 V
= exp
-1.24 V x 2
0.0257 V
K = 1.23 x 10 -42
19.4
The Effect of Concentration on Cell Emf
D
G =
D
G 0 + RT ln Q
D
G = nFE
D
G 0 = nFE 0
nFE = nFE 0 + RT ln Q
Nernst equation
E = E 0 -
RT nF ln Q
E = E 0 -
0.0257 V n ln Q
At 298
E = E 0 -
0.0592 V n log Q
19.5
Will the following reaction occur spontaneously at 25 0 C if
[Fe 2+ ] = 0.60 M and [Cd 2+ ] = 0.010 M ?
Fe 2+
( aq ) + Cd ( s ) Fe ( s ) + Cd 2+
( aq )
Oxidation: Cd Cd 2+ + 2e -
Reduction: 2e + Fe 2+
E 0 = E 0
Fe /Fe
+ E 0
Cd /Cd
2+
E 0 = -0.44 + -(-0.40)
E 0 = -0.04 V
E
2Fe
=
E =
E 0 n = 2
0.0257 V n ln Q
-0.04 V
E = 0.013
-
0.0257 V
2 ln
0.010
0.60
E > 0 Spontaneous
19.5
Charging a Battery
When you charge a battery, you are forcing the electrons backwards (from the + to the -). To do this, you will need a higher voltage backwards than forwards. This is why the ammeter in your car often goes slightly higher while your battery is charging, and then returns to normal.
In your car, the battery charger is called an alternator. If you have a dead battery, it could be the battery needs to be replaced OR the alternator is not charging the battery properly.
Dry cell
Leclanch é cell
Batteries
Anode: Zn (s) Zn 2+ ( aq ) + 2e -
Cathode: 2NH
+
4
( aq ) + 2MnO
2
( s ) + 2e Mn
2
O
3
( s ) + 2NH
3
( aq ) + H
2
O ( l )
Zn ( s ) + 2NH
4
( aq ) + 2MnO
2
( s ) Zn 2+ ( aq ) + 2NH
3
( aq ) + H
2
O ( l ) + Mn
2
O
3
( s )
19.6
Mercury Battery
Batteries
Anode:
Cathode:
Zn(Hg) + 2OH ( aq ) ZnO ( s ) + H
2
O ( l ) + 2e -
HgO ( s ) + H
2
O ( l ) + 2e Hg ( l ) + 2OH
Zn(Hg) + HgO ( s ) ZnO ( s ) + Hg ( l )
( aq )
19.6
Batteries
Lead storage battery
Anode: Pb (s) + SO 2( aq ) PbSO
4 4
( s ) + 2e -
Cathode: PbO
2
( s ) + 4H + ( aq ) + SO 2-
4
(aq ) + 2e PbSO
4
( s ) + 2H
2
Pb ( s ) + PbO
2
( s ) + 4H + ( aq ) + 2SO 2( aq ) 2PbSO
4 4
( s ) + 2H
2
O ( l )
O ( l )
19.6
Batteries
Solid State Lithium Battery
19.6
Batteries
A fuel cell is an electrochemical cell that requires a continuous supply of reactants to keep functioning
Anode:
Cathode:
2H
2
( g ) + 4OH ( aq ) 4H
2
O ( l ) + 4e -
O
2
( g ) + 2H
2
O ( l ) + 4e 4OH ( aq
2H
2
( g ) + O
2
( g ) 2H
2
O ( l )
)
19.6
Corrosion
19.7
Cathodic Protection of an Iron Storage Tank
19.7
Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur.
19.8
Electrolysis of Water
19.8
Chemistry In Action: Dental Filling Discomfort
2+
Hg
2
/Ag
2
Hg
3
0.85 V
2+
Sn /Ag
3
Sn -0.05 V
2+
Sn /Ag
3
Sn -0.05 V
Electrolysis and Mass Changes charge (Coulombs) = current (Amperes) x time (sec)
1 mole e = 96,500 C = 1 Faraday
1 amp = 1 Coulomb / sec
19.8
How much Ca will be produced in an electrolytic cell of molten CaCl
2 if a current of 0.452 A is passed through the cell for 1.5 hours?
Anode:
Cathode:
2Cl ( l ) Cl
2
( g ) + 2e -
Ca 2+ ( l ) + 2e Ca ( s )
Ca 2+ ( l ) + 2Cl ( l ) Ca ( s ) + Cl
2
( g )
2 mole e = 1 mole Ca mol Ca = 0.452
C s x 1.5 hr x 3600 s 1 mol e x hr 96,500 C x
1 mol Ca
2 mol e -
= 0.0126 mol Ca
= 0.50 g Ca
19.8