Half Life
advertisement

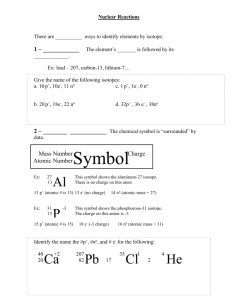
Unit: Nuclear Chemistry Half-Life After today you will be able to… • Identify the factor that nuclear stability is dependent on. • Calculate the half-life for a given radioisotope. • Calculate how much of a radioisotope remains after a given amount of time. Nuclear Stability • Close to 2,000 different nuclei are known. • Approximately 260 are stable and do not decay or change with time. • The stability (resistance to change) depends on its neutron:proton ratio. Nuclear Stability • Plotting a graph of number of neutrons vs. number of protons for each element results in a region called the band of stability. Nuclear Stability • For elements with low atomic numbers (below 20) the ratio of neutrons:protons is about 1. 12 –Example: 6 C (6n/6p = 1) Nuclear Stability • For elements with higher atomic numbers, stable nuclei have more neutrons than protons. The ratio of n:p is closer to 1.5 for these heavier elements. – Example: 206 82 Pb 124n/82p = approx. 1.5 Nuclear Stability • The neutron:proton ratio iswhy Ever wonder important because it some atomic masses determines listed the type of Periodic decay on the that occurs.Table have ( ) around • All nuclei that have an atomic them? Because their number greater thanmasses 83 areare atomic radioactive. estimated due to radioactive decay! Half-Life Half-life: (t1/2) the time required for half of the nuclei of a radioisotope sample to decay to products. • Example: If you have 20 atoms of Radon222, the half life is ~4 days. How many atoms remain at the end of two half lives? 0 t1/2 20 atoms initially 4 days 1 t1/2 10 atoms 8 days 2 t1/2 5 atoms remain Half-Life • We can represent halflife graphically as well. —Example: Carbon-14 However, very seldom do we count atoms. Therefore it is more appropriate to calculate amount that remains in terms of mass. Half-Life • Example: Carbon-14 emits beta radiation and decays with a half-life (t1/2) of 5730 years. Assume you start with a mass of 2.00x10-12g of carbon-14. a.How long is three half-lives? b.How many grams of the isotope remain at the end of three half-lives? a. 3(5730) = 17,190 years b. 2.00x10-12g x 1/2 x 1/2 x 1/2 = 2.5x10-13g Half-Life b. How many grams of the isotope remain at the end of three half-lives? Alternatively, part b can also be calculated like this: 0 t1/2 2.00x10-12g initially x 1/2 1 t1/2 1.00x10-12g x 1/2 2 t1/2 x 1/2 5.00x10-13g 3 t1/2 2.50x10-13g remains Half-Life • Example: Manganese-56 is a beta emitter with a half-life of 2.6 hours. a. How many half-lives did the sample go through at the end of 10.4 hours? b. What is the mass of maganese-56 in a 1.0mg sample of the isotope at the end of 10.4 hours? a. 10.4 h/2.6 h = 4 half-lives b. 1.0mg x 1/2 x 1/2 x 1/2 x 1/2 = 0.063 mg Questions? Complete and turn in the exit ticket.