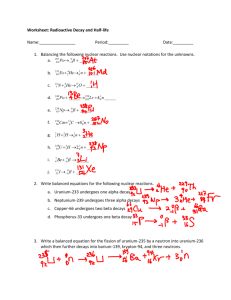
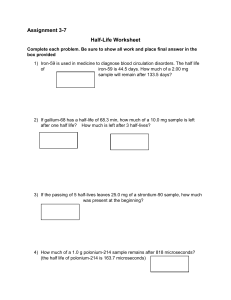
Nuclear Equations Worksheet
advertisement
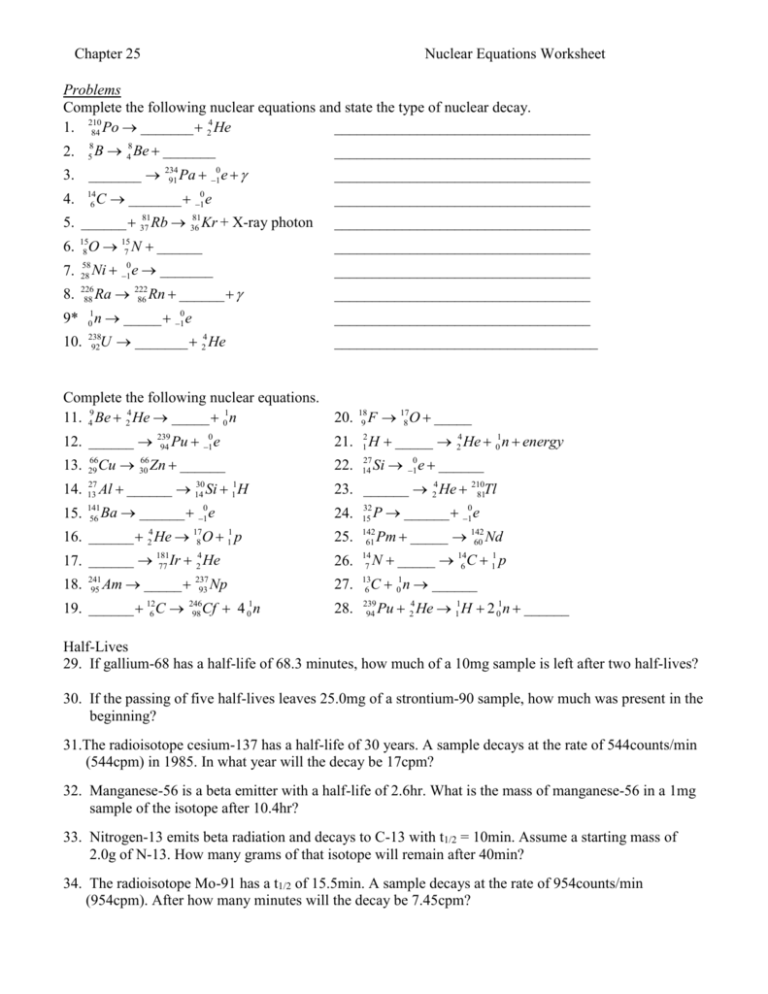
Chapter 25 Nuclear Equations Worksheet Problems Complete the following nuclear equations and state the type of nuclear decay. 4 1. 210 __________________________________ 84 Po _______ 2 He 8 5 2. B 48 Be _______ 3. _______ 234 91 Pa e 0 1 C _______ 10 e 14 6 4. 5. ______ 81 37 Rb 81 36 Kr + X-ray photon 6. O N ______ 15 8 15 7 Ni e _______ 7. 58 28 8. 226 88 0 1 Ra 222 86 Rn ______ n _____ 10 e 9* 1 0 10. 238 92 __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ U _______ He 4 2 ___________________________________ Complete the following nuclear equations. 11. 49 Be 24 He _____ 01n 20. 189 F 178 O _____ 12. ______ Cu 13. 66 29 14. 27 13 15. 141 56 66 30 239 94 Pu 10 e Zn ______ H _____ 24 He 01n energy 21. 2 1 22. 27 14 Si 10 e ______ 30 Al ______ 14 Si 11H 23. ______ 24 He 21081Tl Ba ______ 10 e 24. 32 15 16. ______ 24 He 178 O 11 p 25. 142 61 4 17. ______ 181 77 Ir 2 He 26. 14 7 27. 13 6 28. 239 94 18. 241 95 Am _____ 237 93 Np 19. ______ 126 C Cf n 246 98 P ______ 10 e Pm _____ 142 60 Nd N _____ 146 C 11 p C 01n ______ Pu 24 He 11H 2 01n ______ Half-Lives 29. If gallium-68 has a half-life of 68.3 minutes, how much of a 10mg sample is left after two half-lives? 30. If the passing of five half-lives leaves 25.0mg of a strontium-90 sample, how much was present in the beginning? 31.The radioisotope cesium-137 has a half-life of 30 years. A sample decays at the rate of 544counts/min (544cpm) in 1985. In what year will the decay be 17cpm? 32. Manganese-56 is a beta emitter with a half-life of 2.6hr. What is the mass of manganese-56 in a 1mg sample of the isotope after 10.4hr? 33. Nitrogen-13 emits beta radiation and decays to C-13 with t1/2 = 10min. Assume a starting mass of 2.0g of N-13. How many grams of that isotope will remain after 40min? 34. The radioisotope Mo-91 has a t1/2 of 15.5min. A sample decays at the rate of 954counts/min (954cpm). After how many minutes will the decay be 7.45cpm?