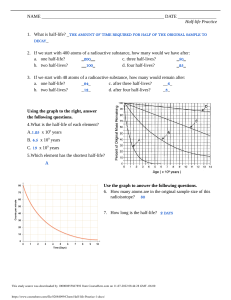
Ch 13 pg 66 1-11
advertisement

Nuclear Chemistry Ch 18, Pg 666, # 1-8 #1. What is meant by the half-life of a radioactive nuclide? #2. Explain how carbon-14 dating is used to determine the age of an object. • (Carbon-14 half life is 5.715 x 103 years = 5,715 years) #3. Why is potassium-40 used to date objects older than 50,000 years old? • (half life Potassium–40 is 1.28 x 109 years) #4. Identify three practical applications of nuclear chemistry 5. What fraction of an original sample of a radioactive isotope remains after three half-lives have passed? #6. How many half-lives of radon222 (half life 3.82 days) have passed in 11.46 days? If 5.2 x 10-8 g of radon-222 remain in a sealed box after 11.46 days, how much was present in the box initially? #7. The half-life of proactinium-234 in its ground state is 6.69 h. What fraction of a given amount remains after 26.76 h? #8. The half-life of thorium-227 is 18.72 days. How many days are required for ¾ of a given amount to decay?