Chemical Bonding Notes - Highline Public Schools
advertisement
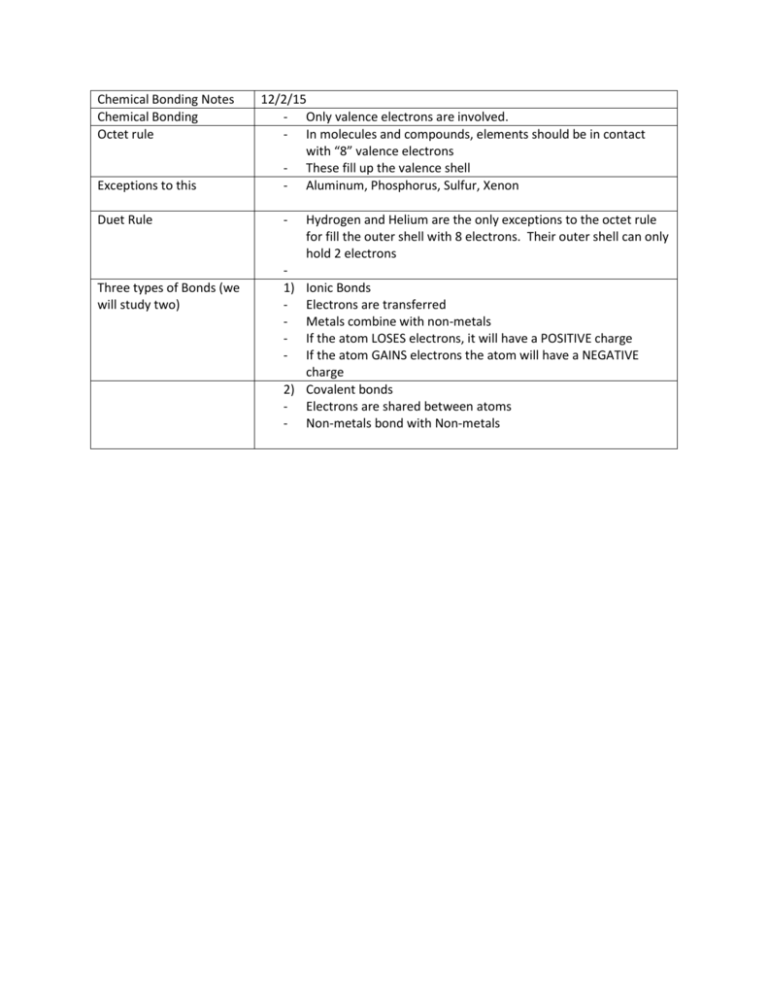
Chemical Bonding Notes Chemical Bonding Octet rule Exceptions to this Duet Rule Three types of Bonds (we will study two) 12/2/15 - Only valence electrons are involved. - In molecules and compounds, elements should be in contact with “8” valence electrons - These fill up the valence shell - Aluminum, Phosphorus, Sulfur, Xenon - 1) - Hydrogen and Helium are the only exceptions to the octet rule for fill the outer shell with 8 electrons. Their outer shell can only hold 2 electrons Ionic Bonds Electrons are transferred Metals combine with non-metals If the atom LOSES electrons, it will have a POSITIVE charge If the atom GAINS electrons the atom will have a NEGATIVE charge 2) Covalent bonds - Electrons are shared between atoms - Non-metals bond with Non-metals