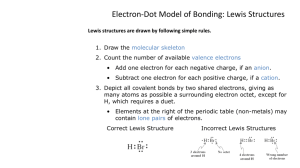
Lewis Structures – Covalent Bonds HBr SCl2 MgBr2 PBr3 NI3 BF3
advertisement

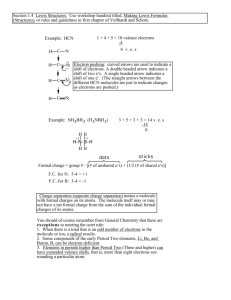
Lewis Structures – Covalent Bonds 1. Determine the types and numbers of atoms in the molecule 2. Write the electron-dot notation for each type of atom in the molecule. 3. Determine the total number of valence electrons in the atoms to be combined. 4. Arrange the atoms to form a skeleton for the molecule. - If carbon is present, it is the central atom. Otherwise, the least-electronegative atom is central (except for hydrogen, which is never central) 5. Add unshared pairs of electrons so that each atom is surrounded by 8 electrons (remember the exceptions) 6. Count the electrons to be sure that the number of valence electrons used equals the number available. - If too many electrons have been used, subtract one or more lone pairs to form multiple bonds until the total number of valence electrons is correct Determine the Lewis structure for the following molecules: HBr SCl2 MgBr2 PBr3 NI3 BF3 N2H2 C2HCl CO2 CHN