9 BRONZE REVISION
advertisement

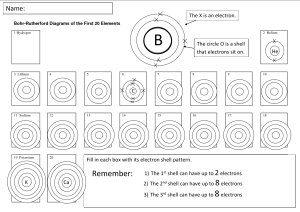
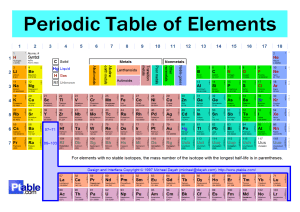
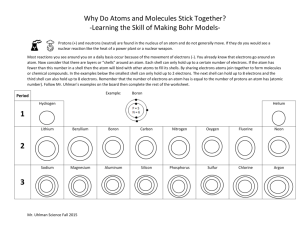
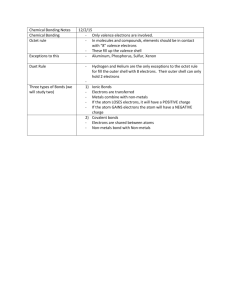
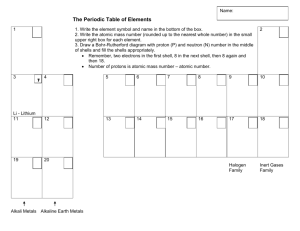
9 BRONZE REVISION – FRIDAY 25th May 2012 – PERIOD 1 THE TEST IN PERIOD 2 TODAY IS ONLY ON CHAPTER 1 ONLY. Read through the chapter. Structure of the atom ( diagram 1.1.1) Atomic and mass numbers and the way to use these to work out the number of protons, electrons and neutrons in the atom. Compounds molecules and lattices. Know an example of each. Mixtures, give examples. Be able to identify the names of common compounds by their formula, eg. Question 24 of review questions on page 6. Know the maximum number of electrons in shell 1, 2 and 3. Use this information to write out this information, eg. An element with 15 electrons is written 2.8.5; 2 electrons in shell 1, 8 electrons in shell 2 and the remaining 5 electrons in shell 3. Group VIII elements are noble gases, know two examples.