ppt
advertisement

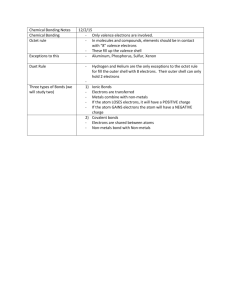
EXCEPTIONS TO THE OCTET RULE Learning Goal: To learn about the lies we’ve been telling you EXCEPTIONS TO THE OCTET Some have too few electrons to form an octet: H, Be, and B (n=1 only needs 2 electrons to complete the energy level) (B is just special) SOME HAVE TOO MANY…. Elements in periods greater than period 3 on the February 25, 20 periodic table have a d orbital available with the same primary quantum number. Atoms in these periods may follow that octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. 4.1 Lewis for Rule Breakers.notebook EXAMPLES OF THE SECRETS WE’VE KEPT FROM YOU: This is because it is possible to excite the sulfur atom sufficiently to push valence atoms into the d orbital to allow molecules such as SF4 and SF6. The sulfur atom in SF4 has 10 valence electrons and 12 valence electrons in SF6. MORE EXAMPLES OF THE SECRETS WE’VE KEPT FROM YOU…. Most commonly occurs with: P, S, Cl, Br, I…but there are others…. Mar 25-2:21 PM OKAY…THIS MIGHT BLOW YOUR MIND… Some noble gases will form compounds….. So…you need to look at your PT and look at the different possible oxidation states…these will tell you bonding options: P3P= 3 bonding electrons P4P = 4 bonding electrons P5P = 5 bonding electrons THERE IS ONE MORE EXCEPTION – FREE RADICALS Free radicals contain at least one unpaired electron in their valence shell. These tend to be very reactive and can form chain reactions. TIME TO PRACTICE… Draw a Lewis Diagram…. phosphorus pentafluoride bromine pentachloride Xenon tetrafluoride Sulfur hexafluoride