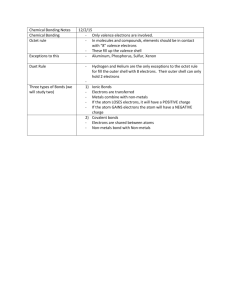
Lewis Dot notes
advertisement

#1 Count the total number of valence electrons in the molecule C: 4 electrons x 1 atom = 4 valence electrons H: 1 electron x 4 atoms = 4 valence electrons Total = 8 valence electrons #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 atoms = 8 octet electrons Total = 16 octet electrons Rules: Hydrogen always has 2 octet electrons Beryllium always has 4 octet electrons Boron wants 6 for neutral molecules (8 if it’s an anion) #3 Subtract the number of valence electrons from the number of octet electrons to find the number of bonding electrons. 16 – 8 = 8 bonding electrons #4 Divide the number of bonding electrons by 2 to find the number of bonds in the molecule. 8 / 2 = 4 bonds in CH4 Why 2? Because there are two electrons in every covalent bond! #5 Draw an arrangement of atoms that has the number of bonds you found in step 4. Of course, there are more rules… Hydrogen and Halogens = bond once! Oxygen’s family and Beryllium bond twice in neutral molecules Nitrogen’s family and Boron bond 3 times in neutral molecules Carbon’s family = bonds 4 times! More hints… If you bond everything together and you have bonds left over, look for double/triple bonds. The atom nearest to the left side of the periodic table is probably in the middle of the molecule. H2O CCl4 NH3 CO2 PCL3 N2 CH4 C2H6 CH3OH BH3 PBr3 N2H2 C2H2 SiH4 C2H4 HF HCl CCl2F2