File
advertisement

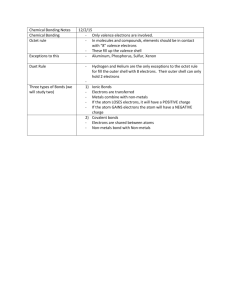
How to draw Lewis Structures for molecular compounds: Step 1: Arrange atoms and electrons 1. Add up total valence electrons (add more for anions, subtract some for cations). 2. Pick central atom (least electronegative). Always carbon; never hydrogen. Usually the first element listed. 3. Place noncentral atoms around central atom and connect using lines to simulate bonds. Subtract electrons used in bonds from total valence electrons. 4. Distribute remaining electrons around the noncentral atoms. Place extra electrons on central atom. Step 2: Check your structure 5. Modify so that the octet rule is met (double and triple bonds, if possible) Some elements can have an expanded octet (OK with more than 8 electrons) while some elements like B and Be are okay with less than an octet (B-6 electrons, Be-4 electrons). 6. Rearrange structure so that all formal charges are as close to (0) as possible. Calculate the formal charge using: FC= Valence - # of bonds – lone electrons (HELP: If two atoms are attached with opposite charges (+ and -), move a pair of electrons from the negative atom into a double bond. If you are dealing with an anion, the negative FC should be on the most electronegative element. If you are dealing with a cation, the positive RC should be on the least electronegative.) 7. Resonance? Are there two of the same atom that look different? If yes, resonance! PRACTICE PROBLEMS: 6. IOF5 12. PCl2111. SCl2 7. N3 13. H2SO4 (hint: do sulfate 2. SiF4 8. SOF4 first, then add H) 3. Ethene 9. SO3214. BrO334. CS2 10. OCCl115. Ethanoic acid (acetic + 5. IO3 11. Pentane acid/vinegar) Give: lewis structure, formal charges, resonance, # of sigma and pi bonds, hybridization, shape name, angles, polar/nonpolar 1. SCl2 (bent) 2. SiF4 (tetrahedral) 3. IO3+(trig planar) 4. CS2 (linear) 5. IOF5(oct) 6. N31- (linear, res) 7. SOF4 (trig bypyr) 8. SO32- (trig pyr, res) 9. OCCl1- (linear) 10. PCl21- (bent, res) 11. BrO33- (t-shape) 12. H2SO4 (hint: do sulfate first and then place H+ in the most logical location)