PPT
advertisement

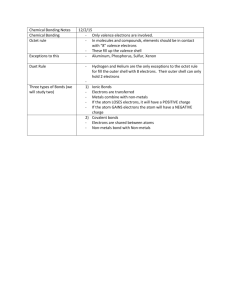
GSCI 163 Lecture 5 Review • Electrons in an atom are distributed in shells, orbitals and energy levels. • When electrons absorb photons they jump to higher orbitals • They move back up by emitting a photon whose energy correspond to the change in energy level Activity • Finding the emission lines of different compounds. Chemistry • Electrons in an atom are distributed in shells, orbitals and energy levels. • The way electrons are shared will determine how elements combine with each other to form compounds. • The highest or last shell of an atom is the valence shell • The valence shell determines the chemistry and properties The periodic table Various groups • Metals and non-metals – Metals tend to lose electrons in chemical reactions – Non-metals tend to gain (or share) electrons in chemical reactions Semi-metals have both metallic and non-metallic properties – Most reactive metal Cesium – Most reactive non-metal Fluorine Noble gases • The valence shell is full. Thus they almost never react. – Argon gas (Ar) is used as an inert gas in light bulbs to prevent the filament, made of tungsten (W), from reacting under intense heat Other families • Alkali metals – Only one valence electron; very soft metals – React so easily with Oxygen (O) and moisture that they need to be stored under oil • Alkaline earth metals – Two valence electrons; harder than alkali metals – Not so reactive. • Halogens – Seven electrons in the valence shell – Very active non-metals • • • • Fluorine – highly corrosive, Chlorine – purifying agent, Bromine – desinfectant Semi-metals (semi-conductors) – 3,4 or 5 electrons in the valence shell – Makes them behave both as metals and insulators Atomic size • Increase when: – Add a new shell (moving down a group) since electrons are farther away from the nucleus – Number of protons decrease (across a period) since electrons are more loosely bound by electrostatic force Cs ~ 0.47 nm He ~ 0.064 nm Ionization energy • Energy required to remove one electron from the outer shell Hardest elements to remove one electron from He H Octet rule • Most common elements have electrons on the s and p orbitals of their outmost shells • We can fit 2 electrons on s and 6 on p, with a total of 8 electrons Octet rule: atoms will combine with other atoms in such a way that gives a full shell of 8 electrons Naming compounds • We represent compounds with a chemical formula: Symbol of the element H2O Number of atoms of the element • Names are also used to identify the compound unambiguously Compounds with special names There are no rules for these. Their names are learned individually Metal and a non-metal • Groups 1A, 2A plus Aluminum (Al), Zinc (Zn) and Silver (Ag). They form only one ion. • Rule for binary compounds: – Name of the metal + non-metal with ending –ide Examples: NaCl – Sodium Chloride Al2O3 – Ca3N2 – Two non-metals • Rule: – The less metallic element (farther left and/or farther down the periodic table) comes first. The second is named with ending –ide . – For more than one element use Greek prefixes: di(2), tri(3), tetra(4), penta (5), hexa (6), hepta (7), octa (8). Examples: HCl – hydrogen chloride CS2 – PBr3 – IF7 – Compounds with polyatomic ions • Metal plus a polyatomic ion: Rule: name of the metal + the name of of the polyatomic ion Example: ZnSO4 – Zinc Sulfate NaC2H3O2 – Mg(NO3)2 – K3PO4 – Next class Calculating reactions • To prepare for the class read: – Handout pages 17 to 20 (day 5) – Presentation by Rebecca Cross, Acids and Bases • To prepare for the quiz read: – Handout pages 16 (day 4) – Power point for this class – Your class notes