Cu 2+ + 2e - Solon City Schools
Electrochemistry Terminology
Oxidation – A process in which an element attains a more positive oxidation state
Na(s) Na + + e -
Reduction – A process in which an element attains a more negative oxidation state
Cl
2
+ 2e 2Cl -
Oxidizing agent -The substance that is reduced is the oxidizing agent
Reducing agent - The substance that is oxidized is the reducing agent
LEO says GER
Electrochemistry Terminology
Anode The electrode where oxidation occurs
Cathode The electrode where reduction occurs
Leo is a
Red uction at the
Cat hode
Balancing Equations
Step 1:
Step 2:
Step 3:
Balancing Equations
e-
2e-
Balancing Equations
Step 4: Multiply each half-reaction by a factor so that the reducing agent supplies as many electrons as the oxidizing agent requires.
Reducing agent
Oxidizing agent
Cu ---> Cu 2+ + 2e-
2 Ag + + 2 e- ---> 2 Ag
Step 5: Add half-reactions to give the overall equation.
Cu + 2 Ag + ---> Cu 2+ + 2Ag
The equation is now balanced for both charge and mass.
Balancing Equations
Balance the following in acid solution—
VO
2
+ + Zn ---> VO 2+ + Zn
Step 1: Write the half-reactions
2+
Ox Zn ---> Zn 2+
Red VO
2
+ ---> VO 2+
Step 2: Balance each half-reaction for mass.
Ox Zn ---> Zn 2+
Red
2 H + + VO
2
+ ---> VO 2+ + H
2
O
Add H
2
O on O-deficient side and add H + other side for H-balance.
on
Balancing Equations
Step 3: Balance half-reactions for charge.
Ox Zn ---> Zn 2+ + 2e-
Red e+ 2 H + + VO
2
+ --> VO 2+ + H
2
O
Step 4: Multiply by an appropriate factor.
Ox Zn ---> Zn 2+ + 2e-
Red 2 e- + 4 H + + 2 VO
2
+ ---> 2 VO 2+ + 2 H
2
O
Step 5: Add balanced half-reactions
Zn + 4 H + + 2 VO
2
+ ---> Zn 2+ + 2 VO 2+ + 2 H
2
O
Balancing Equations for Redox Reactions
A great example of a thermodynamically spontaneous reaction is the thermite reaction. Here, iron oxide (Fe
2
O
3
= rust) and aluminum metal powder undergo a redox ( reduction-oxidation ) reaction to form iron metal and aluminum oxide (Al
2
O
3
= alumina):
Fe
2
O
3
(s) + Al(s) ↔ Al
2
O
3
(s) + Fe(l)
Fe = +3 Al = 0 Al = +3 Fe = 0
Tips on Balancing Equations
How many electrons are transferred in the
2ClO
3
– following reaction?
+ 12H + + 10I – → 5I
2
+ Cl
2
+ 6H
2
O
1) 12
2) 5
3) 2
4) 30
5) 10
43%
14% 14%
0%
2
30
29%
12
5
10
Which of the following reactions is possible at the anode of a galvanic cell?
1. Zn → Zn 2+ + 2e –
2. Zn 2+ + 2e – → Zn
3. Zn 2+ + Cu → Zn + Cu 2+
4. Zn + Cu 2+ → Zn 2+ + Cu
5. two of these
Which of the following species cannot function as an oxidizing agent?
1. S(s)
2. NO
3
– (ag)
3. Cr
2
O
7
2– (aq)
4. I – (aq)
5. MnO
4
– (aq)
62%
8%
0%
31%
0%
S(s
)
N
O3
–(a g)
C r2
O7
2–
(a q)
I–
(a q)
Mn
O4
–
(a q)
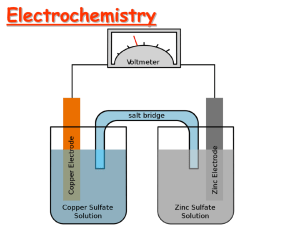
Electrochemical Cells
• An apparatus that allows a redox reaction to occur by transferring electrons through an external connector.
• Product favored reaction ---> voltaic or galvanic cell ----> electric current
• Reactant favored reaction ---> electrolytic cell ---> electric current used to cause chemical change.
Batteries are voltaic cells
Basic Concepts of Electrochemical Cells
Anode Cathode
Terms Used for Voltaic Cells
CELL POTENTIAL, E potential +1.10 V
STANDARD CELL POTENTIAL, E o
Calculating Cell Voltage
Zn(s) ---> Zn 2+ (aq) + 2e-
Cu 2+ (aq) + 2e- ---> Cu(s)
--------------------------------------------
Cu 2+ (aq) + Zn(s) ---> Zn 2+ (aq) + Cu(s)
Measuring
Standard
Electrode
Potential
Potentials are measured against a hydrogen ion reduction reaction, which is arbitrarily assigned a potential of zero volts .
Table of
Reduction
Potentials
Measured against the
Standard
Hydrogen
Electrode
TABLE OF STANDARD
REDUCTION POTENTIALS oxidizing ability of ion Eo (V)
Cu2+ + 2eCu
2 H+ + 2e-
Zn2+ + 2e-
To determine an oxidation from a reduction table, just take the opposite sign of the reduction!
H
2
Zn
+0.34
0.00
-0.76
reducing ability of element
Zn/Cu Electrochemical Cell
+
Anode, negative, source of electrons
Cathode, positive, sink for electrons
Zn(s) ---> Zn 2+ (aq) + 2e-
Cu 2+ (aq) + 2e- ---> Cu(s)
E o = +0.76 V
E o = +0.34 V
---------------------------------------------------------------
Cu 2+ (aq) + Zn(s) ---> Zn 2+ (aq) + Cu(s) E o = +1.10 V
E o for a Voltaic Cell
All ingredients are present. Which way does reaction proceed?
E o for a Voltaic Cell
Day 2 (it’s electric!)
More About
Calculating Cell Voltage
Assume I ion can reduce water.
2 H
2
O + 2e---> H
2
2 I ---> I
2
+ 2e-
+ 2 OH -
-------------------------------------------------
2 I + 2 H
2
O --> I
2
+ 2 OH + H
2
Cathode
Anode
-1.363 V
Negative E˚ means rxn. occurs in opposite direction (the connection is backwards or you are recharging the battery)
Galvanic (Electrochemical) Cells
Spontaneous redox processes have:
A positive cell potential, E 0
A negative free energy change, ( G)
Zn - Cu
Galvanic
Cell
From a table of reduction potentials:
Zn 2+ + 2e Zn E = -0.76V
Cu 2+ + 2e Cu E = +0.34V
Zn - Cu
Galvanic
Cell
The less positive, or more negative reduction potential becomes the oxidation…
Zn Zn 2+ + 2e E = +0.76V
Cu 2+ + 2e Cu E = +0.34V
Zn + Cu 2+ Zn 2+ + Cu E 0 = + 1.10 V
Line
Notation
An abbreviated representation of an electrochemical cell
Zn(s) | Zn 2+ (aq) || Cu 2+ (aq) | Cu(s)
Anode material
| Anode solution
|| Cathode solution
Line notation is cool, just like AC
| Cathode material
Zn
(s)
| Zn 2+
(aq) (1.0M)
|| H +
(aq) (1.0M)
|H
2(g)
(1.00 atm)
| Pt
(s)
Calculating G 0 for a Cell
G 0 = -nFE 0 n
= moles of electrons in balanced redox equation
F
= Faraday constant = 96,485 coulombs/mol e -
Zn + Cu 2+ Zn 2+ + Cu
E 0
= + 1.10 V
G
0 (
2 mol e
)(
96 485 coulombs
)(
1
.
10 mol e
Joules
Coulomb
)
G
0
212267 Joules
212 kJ
The Nernst Equation
Standard potentials assume a concentration of 1.0 M.
The Nernst equation allows us to calculate potential when the two cells are not 1.0 M.
E
E
0
RT ln( Q ) nF
R
= 8.31 J/(mol K)
T
= Temperature in K n
= moles of electrons in balanced redox equation
F
= Faraday constant = 96,485 coulombs/mol e -
Nernst Equation Simplified
At 25 C (298 K) the Nernst Equation is simplified this way:
E
E
0
0 .
0591 log( Q ) n
Equilibrium Constants and Cell Potential
At equilibrium, forward and reverse reactions occur at equal rates, therefore:
1. The battery is “dead”
2. The cell potential,
E
, is zero volts
Modifying the Nernst Equation (at 25 C):
0 volts
E
0
0 .
0591 log( K n
)
Calculating an Equilibrium Constant from a Cell Potential
Zn + Cu 2+ Zn 2+ + Cu
E 0
= + 1.10 V
0 volts
1 .
10
( 1 .
10 )( 2 )
log( K )
0 .
0591
0 .
0591 log( K
2
)
37 .
2
log( K )
10
37 .
2
K
1 .
58 x 10
37
???
Concentration
Cell
Both sides have the same components but at different concentrations.
Step 1: Determine which side undergoes oxidation, and which side undergoes reduction.
Anode
???
Cathode
Concentration
Cell
Both sides have the same components but at different concentrations.
The 1.0 M Zn 2+ must decrease in concentration, and the 0.10 M Zn 2+ must increase in concentration
Zn 2+ ( 1.0M
) + 2e Zn (reduction)
Zn Zn 2+ ( 0.10M
) + 2e (oxidation)
Zn 2+ ( 1.0M
) Zn 2+ ( 0.10M
)
Anode
???
Concentration Cell
Cathode
Concentration
Cell
Both sides have the same components but at different concentrations.
Step 2: Calculate cell potential using the Nernst
Equation (assuming 25 C).
Zn 2+ ( 1.0M
) Zn 2+ ( 0.10M
)
E
E
0
0 .
0591 log( n
Q )
Nernst Calculations
Zn 2+ ( 1.0M
) Zn 2+ ( 0.10M
)
E
0
0 .
E
E
0
0 .
0591 log( n
Q )
0
Volts
n
2 Q
( 0 .
10 )
( 1 .
0 )
E
0 .
0
0 .
0591
2 log(
0 .
10
1 .
0
)
0 .
030 Volts
Day 3
(dahditdadahditahh…Charge!)
Charging a Battery
When you charge a battery, you are forcing the electrons backwards (from the + to the -). To do this, you will need a higher voltage backwards than forwards. This is why the ammeter in your car often goes slightly higher while your battery is charging, and then returns to normal.
In your car, the battery charger is called an alternator. If you have a dead battery, it could be the battery needs to be replaced OR the alternator is not charging the battery properly.
Dry Cell Battery
Anode (-)
Zn ---> Zn 2+ + 2e-
Cathode (+)
2 NH
4
+ + 2e- ---> 2 NH
3
+ H
2
Alkaline Battery
Nearly same reactions as in common dry cell, but under basic conditions.
Anode (-): Zn + 2 OH ---> ZnO + H
2
O + 2e-
Cathode (+): 2 MnO
2
+ H
2
O + 2e- ---> Mn
2
O
3
+ 2 OH -
Anode:
Cathode:
Mercury Battery
Lead Storage Battery
Anode (-)
Cathode (+)
Ni-Cad Battery
Anode (-)
Cd + 2 OH ---> Cd(OH)
2
Cathode (+)
+ 2e-
NiO(OH) + H
2
O + e- ---> Ni(OH)
2
+ OH -
The positive electrode is made of Lithium cobalt oxide, or LiCoO
2
The negative electrode is made of carbon.
H
2
as a Fuel
Cars can use electricity generated by H
2
/O
2
H
2 fuel cells.
carried in tanks or generated from hydrocarbons
The Electrochemical Corrosion of Iron
Preventing Corrosion
• Coating to keep out air and water.
– Galvanizing - Putting on a zinc coat
• Has a lower reduction potential, so it is more. easily oxidized.
• Alloying with metals that form oxide coats.
• Cathodic Protection - Attaching large pieces of an active metal like magnesium that get oxidized instead.
Cathodic Protection of an
Underground Pipe
Electrolytic
Processes
Electrolytic processes are
NOT spontaneous.
They have:
A negative cell potential, (-E 0 )
A positive free energy change, (+ G)
Electrolysis of
Water
In acidic solution
Anode rxn:
Cathode rxn:
4 H
2
O
2
H
2
4 e
O
O
2
2 H
2
4
H
4 OH
4 e
2 H
2
O
2 H
2
O
2
-1.23 V
-0.83 V
-2.06 V
Electroplating of
Silver
Anode reaction:
Ag Ag + + e -
Cathode reaction:
Ag + + e Ag
Electroplating requirements:
1. Solution of the plating metal
2. Anode made of the plating metal
3. Cathode with the object to be plated
4. Source of current
Calculating plating
Step 1 – convert current and time to quantity of charge in coulombs a. Amps x time = total charge transferred in coulombs
(Coulomb/sec) x sec = coulombs
Step 2 – convert quantity of charge in coulombs to moles of electrons coulombs /(96,485 coulombs/mol e-) = mol e-
Step 3 – Convert moles of electrons to moles of substance mol e- x (mole substance/mol e-) = mol substance
Step 4 – Convert moles of substance to grams of substance mol substance x formula mass of substance = mass of substance
Suppose that in starting a car on a cold morning a current of 125 amperes is drawn for 15.0 seconds from a cell of the type described above.
How many grams of Pb would be consumed?
(The atomic weight of Pb is 207.19.)
Pb 2+ + 2e Pb
125 C 15 sec 1 mol e 1 mol Pb
1 sec 96 485 C 2 mol e -
207.19 g
1 mol Pb
2.01 g Pb
Solving an Electroplating Problem
Q: How many seconds will it take to plate out
5.0 grams of silver from a solution of AgNO
3 using a 20.0 Ampere current?
Ag + + e Ag
5.0 g 1 mol Ag
107.87 g
1 mol e -
1 mol Ag
96 485 C
1 mol e -
1 s
20.0 C
= 2.2 x 10 2 s