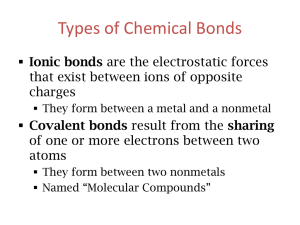
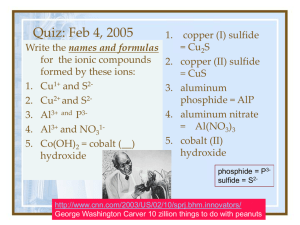
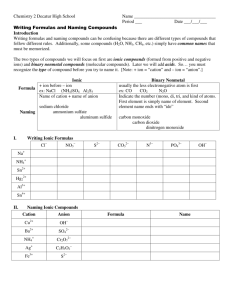
Ch. 5/6 Naming
advertisement
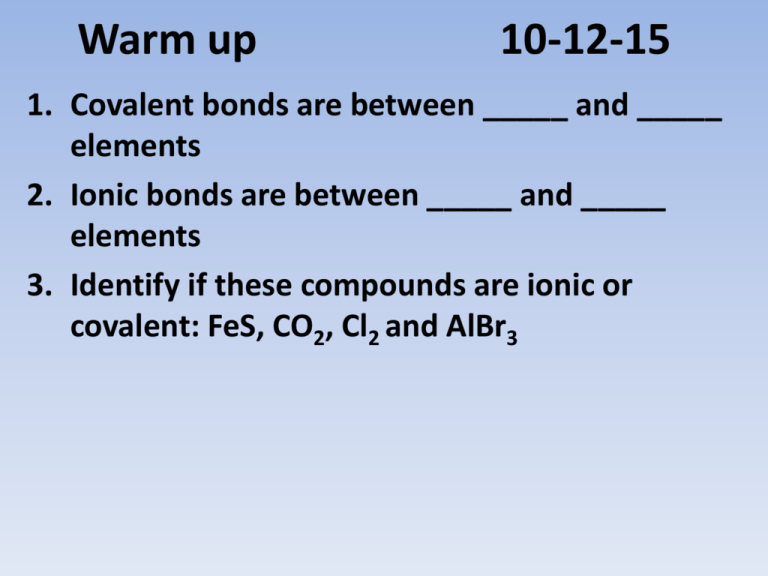
Warm up 10-12-15 1. Covalent bonds are between _____ and _____ elements 2. Ionic bonds are between _____ and _____ elements 3. Identify if these compounds are ionic or covalent: FeS, CO2, Cl2 and AlBr3 Formulas and Names of Ionic and Covalent Compounds Chp 5-3 and 6-2 Nomenclature: Naming of compounds • Must determine which type of bond made up the compound - Ionic bond has metal elements - Covalent bond has no metal elements Naming Covalent (Molecular) Compounds 1. Use a prefix to indicate the amount of each element in the compound 2. Never use mono– on the first element name. 3. Give the last element an –ide ending. Naming Covalent (Molecular) Compounds • Mono– 1 • • • • • • • • • Di– 2 Tri– 3 Tetra– 4 Penta– 5 Hexa– 6 Hepta– 7 Octa– 8 Nona– 9 Deca– 10 Prefixes Examples • CO2 • B2H4 • Dinitrogen trioxide Naming Covalent Compounds Practice Chemical Formula Name 1. CO 2. P2S5 3. SiO2 4. SCl4 5. Trinitrogen pentabromide 6. Dinitrogen trioxide Why incorrect? Monosulfur dioxide Naming Ionic Compounds 1. Ending element change to –ide. (no prefixes) 2. Make sure all the charge cancel each other out 3. If there is a transition metal you indicate the charge with a roman numeral after the name of the metal. 4. If it is a polyatomic ion, you simply use the name as it is. (no –ide) Examples 1. 2. 3. 4. 5. 6. CaCl2 MgI2 Potassium Sulfide Beryllium Fluoride Iron (II) Nitride FeCl2 Naming Ionic Compounds Practice 1. 2. 3. 4. 5. 6. 7. ______________ ______________ Lithium Bromide Calcium Fluoride Aluminum Oxide ____________ Iron (III) Phosphide AlCl3 Na2S _____ _____ _____ CuCl2 ____ Writing Chemical Formula for Ionic Compounds -Write the symbol and charge -Cation goes in front -Make sure all the charges Cancel each other out Transition metal Naming Ionic Compounds - Polyatomic Ions (NH4)+ Ammonium (OH)- Hydroxide (CO3)-2 Carbonate (NO3)- Nitrate (SO4)-2 Sulfate (PO4 )-3 Phosphate (Cr2O7)-2 Dichromate (NH3) Ammonia (NO2)- Nitrite (SO3)-2 Sulfite (PO3)-3 Phosphite Naming Polyatomic Ions 1. 2. 3. 4. 5. 6. 7. 8. ______________ Li2SO4 ______________ Sr(NO3)2 Potassium Phosphate _____ Ammonium Oxide _____ ______________ NaOH ______________ Ca(NO2)2 Calcium Carbonate ______ Ammonium Sulfide _____ More Examples • Mixture of covalent, ionic and polyatomic nomenclature. SrS Strontium Sulfide GaCl3 Gallium Chloride Calcium Oxide CaO Sulfate SO42- Phosphate PO43- Strontium Nitride Sr3N2 Li3N Lithium Nitride Hydroxide OH- Beryllium Carbonate BeCO3 BeSe Beryllium Selenide Mg(NO3)2 Magnesium Nitrate KBr Potassium Bromide AlPO4 Aluminum Phosphate Magnesium Arsenide Mg3As2 NaOH Sodium Hydroxide Potassium Sulfate K2SO4 Ba2P3 Barium Phosphide Rubidium Phosphide Rb3P Potassium Chloride KCl Sodium Oxide Na2O Rb2O Rubidium Oxide