Naming Compounds 2013
advertisement

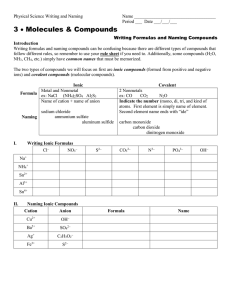
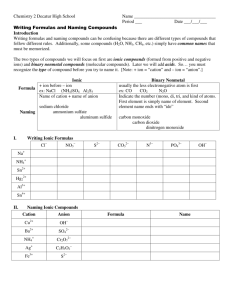
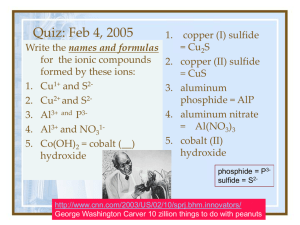
Chemical Names and Formulas Overview •Metals and Non-Metals •Ions and Ionic Charges •Types of Compounds •Systematic Names -Writing Names and Formulas Metals and Nonmetals Stairway Of Division on Periodic Table C, P, Se, I, Rn and to the right are nonmetals B, Si, As, Ge, Sb, Te, At are metalloids All others are metals – http://isbchem1.pbworks.com/w/page/9206122/ Semi-Metals_E_Block Ions Cations Anions Positively Charged Atoms Negatively Charged Atoms i.e. Na + i.e. Cl - TYPES OF COMPOUNDS Ionic Compounds Ionic means a metal and a non-metal (or cation and anion) These elements are NOT attached Writing Formulas • charges must balance so compound charge is neutral. Molecular Compounds • composed of molecules in which elements share electrons. • usually composed of 2 nonmetals. • these elements are attached CHEMICAL FORMULAS Definition: shows the kind and numbers of atoms in the smallest representative unit of the substance. i. e. NaCl WRITING FORMULAS AND NAMING IONIC COMPOUNDS Writing Formulas from Names • 1st word = CATION • 2nd word = ANION name with ide ending. IONIC COMPOUNDS NaBr Sodium MgF2 Bromide Magnesium Fluoride IONIC COMPOUNDS Potassium Chloride Aluminum Oxide • notice ending of name is ide! K + Cl- -> KCl Al3 + O2- -> Al2O3 Polyatomic Ions Definition: tightly bound groups of atoms that behave as a unit and carry a charge. Example: SO32-, NO2-, ClO2- Writing Formulas and Naming Compounds with Polyatomic Ions Ca2 + CO32-> CaCO3 Ca(NO3)2 Calcium Carbonate Calcium Nitrate Naming with Transition Metals First word = CATION Second word = ANION You need to determine what charge is on the transition metal if more than one exists. Naming Transition Metals Copper Oxide (I) Cu2O Writing Formulas with Transition Metals FeCl3 Cu2O FeCl2 Iron (III) Chloride Copper (I) Oxide Iron (II) Chloride MORE ABOUT NAMING COMPOUNDS Your ability to name compounds and write formulas hinges on your ability to recognize whether a compound is Ionic or Molecular. Naming Molecular Compounds Prefixes are used to show how many atoms are present in each molecule. mono, di, tri,tetra, penta, hexa, hepta, octa, nona, deca Writing Molecular Compounds CO2 Carbon Dioxide • No mono prefix is used on first element • Di means two oxygen atoms S4N2 Tetrasulfur Dinitride Naming Molecular Compounds (prefix + element name + root -ide) * No mono prefix is used on first element * The Greek prefixes for 1 through 8 are: mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octo- i. e. CO2 i.e. N2O i.e. PCl3 Carbon Dioxide Dinitrogen Monoxide Phosphorous Trichloride