Legami Chimici e Nomenclatura: Composti Ionici, Covalenti
advertisement
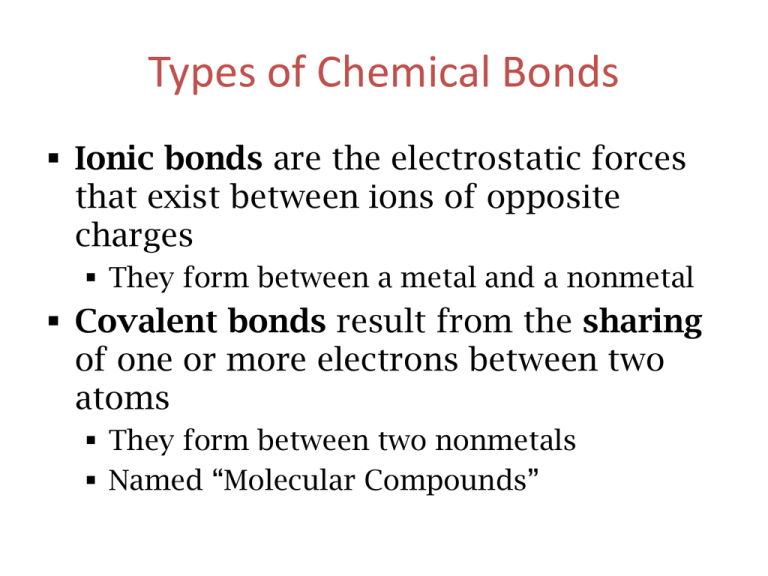
Types of Chemical Bonds Ionic bonds are the electrostatic forces that exist between ions of opposite charges They form between a metal and a nonmetal Covalent bonds result from the sharing of one or more electrons between two atoms They form between two nonmetals Named “Molecular Compounds” Molecular Compounds CO2 Carbon dioxide CH4 methane BCl3 boron trichloride All are formed from two or more nonmetals. Ionic compounds generally involve a metal and nonmetal (NaCl) Identify with contain covalent bonds 1. BaI2 Ionic A. I2O5 Covalent 2. P4S3 Covalent B. Cu(ClO4)2 Ionic 3. Ca(OH)2 Ionic C. CS2 Covalent 4. FeCO3 Ionic D. B2Cl4 Covalent 5. Na2Cr2O7 Ionic Naming Molecular (Covalent) Nomenclature for two nonmetals • Prefix System (binary compounds) 1. Less electronegative atom comes first (which ever is written first in the formula) 2. Add prefixes to indicate # of atoms. a.) Omit mono- prefix on the FIRST element. b.) Mono- is required on the SECOND element. 3. Change the ending of the second element to -ide. Molecular Nomenclature Prefixes You need to memorize these. PREFIX monoditritetrapentahexaheptaoctanonadeca- NUMBER 1 2 3 4 5 6 7 8 9 10 Molecular Nomenclature: Examples • CCl4 •carbon tetrachloride 1. Add prefixes to indicate # of atoms. 1. Monocarbon tetrachloride a.) Omit mono- prefix on the FIRST element. b.) Mono- is required on the SECOND element. Molecular Nomenclature: Examples • CCl4 • carbon tetrachloride • N2O • dinitrogen monoxide • SF6 • sulfur hexafluoride More Molecular Examples • arsenic trichloride • AsCl3 • dinitrogen pentoxide • N2O5 • tetraphosphorus decoxide • P4O10 Learning Check Fill in the blanks to complete the following names of covalent compounds. CO CO2 mon carbon ______oxide dioxide carbon _______________ CCl4 tri phosphorus _______chloride tetra carbon ________chloride N2O di mon _____nitrogen _____oxide PCl3 Learning Check 1. P2O5 a) phosphorus oxide b) phosphorus pentoxide c) diphosphorus pentoxide 2. Cl2O7 a) dichlorine heptoxide b) dichlorine oxide c) chlorine heptoxide 3. Cl2 a) chlorine b) dichlorine c) dichloride A flow chart for naming binary compounds. Mixed Review Name the following compounds: 1. CaO N2O3 a) calcium oxide 3. a) nitrogen oxide b) calcium(I) oxide b) dinitrogen trioxide c) calcium (II) oxide c) nitrogen trioxide 2. SnCl4 a) tin tetrachloride b) tin(II) chloride c) tin(IV) chloride Mixed Practice 1. 2. 3. 4. 5. 6. 7. 8. 9. Dinitrogen monoxide Potassium sulfide Copper (II) nitrate Dichlorine heptoxide Chromium (III) sulfate Iron (III) sulfite Calcium oxide Barium carbonate Iodine monochloride 1. 2. 3. 4. 5. 6. 7. 8. 9. BaI2 P4S3 Ca(OH)2 FeCO3 Na2Cr2O7 I2O5 Cu(ClO4)2 CS2 B2Cl4



