Naming Compounds
advertisement

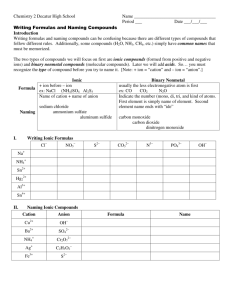
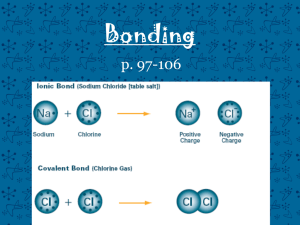
Naming Compounds Ionic and Covalent First thing first… Determine if the compound is Ionic or Covalent. Ionic = Metal + Nonmetal Covalent = Nonmetal + Nonmetal Metal vs Nonmetal Nonmetals Metals Metal vs Nonmetal Nonmetals Metals Metal vs Nonmetal Metalloids Metals Nonmetals Ionic: Binary (2 elements) Positive ion + Negative ion Change ending to “ide” Ionic Naming Subscripts have no affect on the name. Example: NaCl = Sodium Chloride CaCl2 = Calcium Chloride Transition Metals Roman Numerals are used to indicate the CHARGE of transition metals. 1 I 2 3 4 5 6 7 8 9 10 II III IV V VI VII VIII IX X Transition Metal Example Charge of Nickel (+3) Nickel (III) Sulfide Charge of Sulfur (-2) +3 -2 Ni2S3 Polyatomic ions An ion containing more than one atom. Polyatomic ion Families Common Polyatomic Ions NH4+ CNOHNO3NO2SO4-2 SO3-2 PO4-3 ClO3MnO4CO3- ammonium cyanide hydroxide nitrate nitrite sulfate sulfite phosphate chlorate permanganate carbonate Memorizing Polyatomics -1 -1 -2 -2 3 4 3 -3 OH NO SO CO PO hydroxide nitrate sulfate carbonate 4 phosphate Using polyatomics +2 +3 Magnesium Phosphate Mg PO4 Mg3(PO4)2 What to remember about ionic compounds End in “ide” Transition metals Roman Numeral indicates charge Polyatomic ions Try a few Ionic Compounds K3N __________________ CuOH __________________ FePO4 __________________ MgO __________________ Try it the other way Vanadium (III) sulfide Manganese (II) phosphate _______ Sodium oxide Lithium Fluroide _______ _______ _______ Covalent Naming Know your prefixes 1 2 3 4 5 6 7 8 9 10 Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Use the prefixes The prefix indicates the number of atoms of the element Change the ending of the second compound to “ide” Ends in “ide” diphosphorous trioxide 2 Phosphorous atoms 3 Oxygen atoms Use the prefixes If the first element is singular do not use the mono prefix. Ends in “ide” Carbon dioxide 1 Carbon atoms 2 Oxygen atoms Use the prefixes If the second element is singular you must use the mono prefix. Ends in “ide” Carbon monoxide 1 Carbon atoms 1 Oxygen atom Try a few Covalent Compounds NF3 ___________________________ Si2Br6 ___________________________ N2O3 ___________________________ ClO2 ___________________________ CF4 ___________________________ Try it the other way now Hexaboron Silicide Dihydrogen monoxide ______________ Iodine pentafluoride ______________ Phorsphorous triiodide ______________ Carbon tetrachloride ______________ ______________ YAY! You are now an expert at naming compounds