Thermochemistry
advertisement
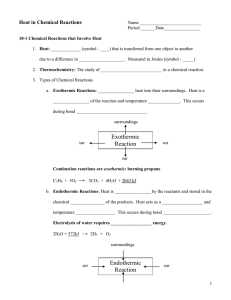
Thermochemistry Key terms Thermodynamics – study of energy and its interconversions Energy – capacity to do work or produce energy Law of conservation of energy – energy can be neither created nor destroyed but only changed in form. Potential energy (PE) – energy due to position or composition Kinetic energy (KE) – energy due to motion Heat – transfer of energy between objects of different temperature Work – force acting over a distance Pathway - the way the energy is transferred State function/state property – depends on current (present) state (ex = energy) Heat transfer Exothermic = energy given off; heat flows out of the system Endothermic = energy absorbed; heat flows into the system Where does the energy come from? – Difference in potential energies between reactants and products – Energy gained by the surroundings must be equal to the energy lost by the system (reaction) – Exothermic reaction - lowers potential energy; reactants higher than products – Endothermic reaction – increase potential energy; reactants lower than products – See energy diagrams Energy Diagrams Exothermic Reaction Endothermic Reaction Reactants Products ∆PE Products • Feels warm • Change in energy negative - ∆E < 0 ENERGY ∆PE ENERGY Reactants • Feels cool • Change in energy positive ∆E > 0 Internal energy in a system – E = sum of potential and kinetic energies of all the particles in the system ∆E = q + w ∆E = change in systems internal energy q = heat w = work (system) Or if we look at surroundings ∆E = q – w’ w’ = work (surroundings view) Units for E = Joules - ? Enthalpy Enthalpy = H (a state property) H = E + PV E = internal energy of system P = pressure of system V = volume of system ∆H = qp At constant pressure, the change in enthalpy (heat of reaction) is equal to energy flow as heat. ∆H = Hproducts – Hreactants ∆H positive = endothermic ∆H negative = exothermic Calorimetry Key Terms Calorimetry – science of measuring heat Calorimeter – device used to determine heat of reaction; p.255 for diagram Constant pressure calorimetry – atmospheric pressure constant used for determining change in enthalpy (heats of reaction) ∆H = qp = q Constant volume calorimetry – use a bomb calorimeter ∆E = qv = q ∆E = ∆T * heat capacity of calorimeter Specific Heat Heat capacity – energy needed to raise temperature 1 degree of substance heat absorbed C= increase in temp J/K or J/°C; depends on amount of substance Specific heat capacity – heat capacity per grams of substance; J/K*g or J/°C*g chart on p.254 Molar heat capacity – heat capacity per mole of substance; J/K*mol or J/°C*mol Equation: q = s x m x ∆T q = C x m x ∆T q= heat (energy) ; J s or C = specific heat (from chart); 𝐽 𝑔°𝐶 m = mass; g ∆T = change in temperature (Tfinal –Tinitial); °C Hess’s Law • Change in enthalpy is same whether occurs in one step or series of steps • Get a net reaction if occurs in series of steps Reaction 1 H1 Reaction 2 H2 . Net reaction ∆H = H1 + H2 • If reverse the reaction, sign of ∆H reversed Standard enthalpy of formation • Change in enthalpy that accompanies formation of 1 mole of a compound from its elements in its standard states; ∆H°f – Under standard conditions/standard states Enthalpy of formation of compounds = chart Enthalpy of formation of an element = 0 Apply Hess’s Law to figure out overall ∆H°f ∆H°f = ∑np∆H°f (products) - ∑nr∆H°f (reactants) Multiply the enthalpies by the coefficients in balanced equation Sources of Energy • Fossil fuels – lead to greenhouse effects – Coal – Petroleum – Natural gas • New energy sources - costly – Coal conversion – Hyodrogen – Alternate fuels • Oil shale • Ethanol • kerogen