Study Guide Chemistry Final 2014 Topics
advertisement

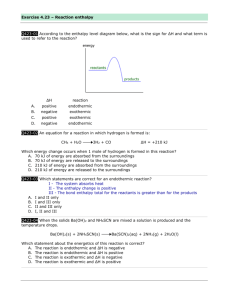
Study Guide Chemistry Final 2014 Topics: This final will cover the topics we studied in the second semester of the 2013-2014 school year. The topics that were covered were: Chemical Reactions; Stoichiometry; Thermochemistry; Gas Laws; and Solutions w/ Acids and Bases. Each of these units has resources on my website that should be used to review and practice for the test. Also other resources you have to help prepare yourself for the test are your notes, labs, previous tests, quizzes and homework. I have posted also podcast and khan academy links to many of the themes covered in this final and website links to practice problems. Please understand that since this test covers the second half of the year, you will need to be comfortable with the first semesters content and practices in order to be successful. Chemical Reactions: You will need to know the five types of chemical reactions: Synthesis, decomposition, single replacement, double replacement, and combustion. You will need to be able to identify the reactions from a chemical equation, to balance the equation, and predict products from known reactants. Important concepts from the first semester such as; ionic bonding, ion charge balancing, polyatomic ions, and balancing chemical formulas will be used. Stoichiometry: Stoichiometry will be integrated throughout the test and will be used in chemical reaction problems, gas law problems and thermochemistry problems on the test. This skill of using conversion factors to solve for changes in chemical systems will be necessary through all aspects of the test. Some typical conversions will be mole to mole, mole to mass, mass to mass, and mole to liter. Please review using the website and previous test and quizzes. Thermochemistry: Parts of the test that will cover topics from thermochemistry will also be asking you to show that you understand stoichiometry and chemical reactions. The key topics that will be covered are solving for specific heat (heat capacity) q= m x cp x ∆T and solving enthalpy problems. Almost all chemical and physical reactions involve energy (usually in the form of heat) being released or added. An exothermic change is a reaction that releases energy. An endothermic change is one in which the energy must be added for the reaction to occur. For exothermic reactions, energy can be thought of as a product in the reaction.For endothermic changes, energy can be thought of as a reactant in the reaction. If a chemical reaction occurs at constant pressure, as all of our chemical reactions do we can consider a property called enthalpy. Enthalpy (H) is the energy (heat) content of a system at constant pressure. You cannot measure the actual energy or enthalpy of a substance, but you can measure the change in enthalpy for a reaction. This change is symbolized by ∆Hrxn. For exothermic reactions, enthalpy values are always negative, that is the energy of the products is lower than that of the reactants. This is because energy is released as new bonds are formed in the products and this amount of energy is greater than the energy required to break the old bonds in the reactants. ∆Hrxn = Hproducts - Hreactants (small # - BIG#) = - negative # For example: 4Fe + 3O2 2Fe2O3 ∆Hrxn = -1625 kJ For endothermic reactions, enthalpy values are always positive, that is that energy of the products is greater than that of the reactions. This is because the energy released as new bonds are formed in the products is less than the energy required to break the bonds in the reactants. This energy must be supplied in order for the reaction to occur. The added energy does not disappear, of course due to the Law of Conservation of Energy. Instead, it becomes stored in the chemical bonds of the products. ∆Hrxn = Hproducts - Hreactants (BIG# - small #) = positive # For example: C + H2O CO + H2 ∆Hrxn = + 113 kJ Gas Laws The gas laws sections of the test will have questions that will test your ability to identify gas laws and manipulate variables for the laws to find how the system can change. The Laws covered were Boyles, Charles, Gay-Lussac, Advogadro, and The Ideal Gas Law. Please be sure you each law, the equation and variables for each and whether they are directly or indirectly proportional. Also you will need to be able to show what happen to molar volumes of gases at Standard Pressure and Temperature.