Thermochem
advertisement

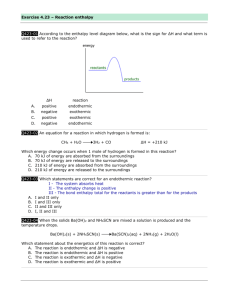
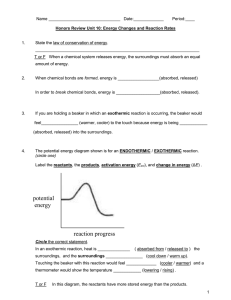
Thermochemistry “The Quick and Dirty” Energy changes accompany every chemical and physical change. In chemistry heat energy is the form of energy that we are most often interested in. Kinetic energy (energy of motion) Potential energy (stored energy) Chemical bond energy is the major form of potential energy we are concerned about in chemistry. Heat transfer is always from the warmer object to the colder object. The standard unit of heat energy is joule (J). Kelvin to 0C + 273 Open/closed systems: Closed systems can exchange energy but not matter. Law of conservation of Energy = 1st Law of Thermodynamics Phase Change Graph For Water Exothermic reaction – molar enthalpy (ΔH) is lost by conversion to heat or light. - energy is lost to the surroundings - energy of the system decreases Endothermic reaction – energy in the surroundings is absorbed and converted to molar enthalpy. Calorimeter – thermally insulated container in which the exchange between the system and its surroundings can be measured q = cmΔT m= q cΔT ΔT = q cm Energy changes during a state change: q = nΔH phase n- number of moles Hf – heat of fusion Hv – heat of vapourization Exothermic Reactions - net release of energy Energy term is on the product side Fe2O3 + 2Al 2Al2O3 + 2Fe + 847.6 KJ Endothermic Reactions – net input of energy Energy term is on the reactants side. 2 SO3 + 198 KJ 2 SO2 + O2 Chemical Reactions occur spontaneously for two reasons: 1. The products of the reaction have less energy than the reactants (burning a match) always exothermic. 2. Products are more random than the reactants. This is the entropy (S). Changes that produce substances with greater randomness (+ΔS) are favoured in nature and drive the reaction to occur. More Randomness: 1. Solid state liquid 2. Liquid state gas 3. Solid gas 4. Formation of a mixture 5. Increase in volume of a gas Gas Highest S Aqueous Liquid Solid Lowest S Enthalpy: Δ H = H final – H initial OR Δ H = H products – H reactants Exothermic: -ΔH Endothermic: +ΔH The reactants have less potential energy than do the products. Energy must be input in order to raise the particles up to the higher energy level. Energy + A + B --> AB The reactants have more potential energy than the products have. The extra energy is released to the surroundings. A + B --> AB + Energy Writing Equations: 1. ΔH notation for 1 mol of CO exothermic Fe2O3(s) + 3CO(g) 3CO2(g) + 2 Fe (s) + 25 kJ 1/3 Fe2O3(s) + CO(g) CO2 (g) + 2/3 Fe(s) 2. Using Energy as a term: 3FeCl3(s) 3FeCl2(s) + 3/2 Cl2 ΔH = -8.3 kJ endothermic ΔH = + 173 kJ 6 FeCl3(s) + 346 kJ 6 FeCl2(s) + 3Cl2(g) Calculating Heat: How much heat is produced when 95 g of methane is burned in oxygen? CH4 (g) + 2 O2(g) CO2(g) + 2H2O(g) 95g x 1mol = 5.9 mol 16g ΔH = 891 kJ/mol 1 mol so 891 kJ q= nΔH = 5.9 mol x 891 kJ/mol = 5300 kJ ΔH = -891kJ Calorimetry ΔH substance = mcΔT n m -mass of water (usually taking in heat) C -specific heat of water 4.18 J/goC ΔT – change in temperature n – moles of the substance that you are calculating the ΔH of Hess’s Law Based on 1 mole. Reaction occurs in a series of steps in which the intermediates are cancelled. OR ΔH reaction = ΣHf products –ΣHf products Standard Heat of Formations from elements( you will need to write a balanced equation for the formation of the substance). Bond Energies – uses Lewis Structures to draw structural formulas (energy values come from table) ΔH = Σ Reactants – Σproducts ΔG: - ΔG reaction is spontaneous