Study Guide for Exam 3 C341 Fall 2013 Important Information: 7:15
advertisement

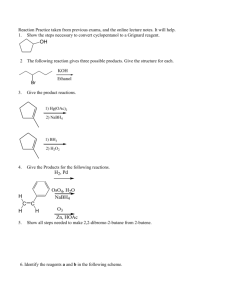
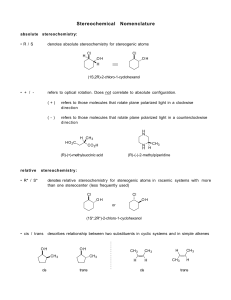
Study Guide for Exam 3 C341 Fall 2013 Important Information: 7:15-9:15PM, Tuesday, December 3 Bring your student ID Show up 10-15 minutes early You may use models, but you may not bring model instructions No calculators! No phones! Anything else you bring must be in a sealed bag under your chair Room Assignments: Last name A-B in Psychology 101 C-J in Rawles 100 K-MO in Jordan A100 MP-SC in Woodburn 120 SD-Z in Radio and TV 251 DSS students: If you have provided the instructor with DSS documentation, please report to Education Building room 1230 at 5:15PM for your exam. Please confirm via email with the instructor if you will be using this option for exam 3. Early exams: No one will be permitted to take an exam outside the regularly scheduled room without PRIOR INSTRUCTOR PERMISSION. If you attempt to do so, you will not be permitted, and you will receive a zero for the exam. Exam Content: Chapters 9, 10, 12. (Concepts from chapters on stereochemistry, substitutions, and eliminations are all applied, but there won’t be problems directly from those chapters.) All material covered in class, homework, or discussion sections could be on the exam. Some major topics include: Nomenclature of alkenes and alkynes, Reactions of alkenes (mechanism, regiochemistry, Markovnicov, anti-Markcovnicov, carbocation stability, transition states, stereochemistry, anti addition, syn addition, predict the product, provide reagents), Reactions of alkynes (mechanism, enol formation under acid, enol formation under basic, regiochemistry, stereochemistry of partial reductions, alkylation of alkynes); multistep synthesis Suggestions for studying: First, review daily homework and discussion sheets, especially problems you initially missed Next, work through additional problems given on last page of syllabus Use the practice problems on the following page and the practice exam posted on the website 1. (14pts) Draw a mechanism to account for this reaction, including all arrows, intermediates, and resonance structures. How does your mechanism explain the observed regiochemistry of the product? How does your mechanism explain why the reaction is an anti addition? 2. (16pts) Predict the major product(s) of 4 of the following 5 problems. Clearly mark the one that you do not want graded or the first 4 will be graded. Include proper stereochemistry, and indicate if both enantiomers are formed. 3. (4pts) The following reaction breaks the Markovnikov rule. Use the mechanism to explain why the anti-Markovnicov product forms in this case. 4. (16pts) Provide the missing starting materials or reagents for 4 of the following 5 problems. Clearly mark the one that you do not want graded or the first five will be graded. 5. (5pts) Provide a mechanism for this reaction. 6. (12pts) Provide all the reagents necessary for these multistep reactions.