Quiz Maker
advertisement

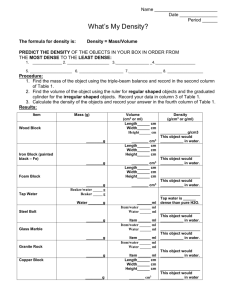
Earth Science Name______________________________ Chapter 0 Period______________________________ Worksheet Date_______________________________ ___________________________________________________________________________________ Density Background An important property of matter which scientists use in distinguishing one substance from another is density. Density is the mass of a certain volume of a substance. That volume is usually 1 milliliter (mL). The mass we will be using in class is the metric standard called the gram (g). Density can also be defined as the number of grams of a substance for a volume of 1 milliliter (g/mL). Solids, liquids, and gases all have their own density. The densities of some common materials are listed below. In order to find the density of any object or substance you need only to divide the mass of the substance by the volume of that substance in milliliters (mL) or cubic centimeters (cm3). Mass (g) Density = ----------------------------Volume (mL or cm3) Comparing Densities (0.25 points each) Study the list of densities of some common materials below and answer the questions that follow. 1. Which substance is the most dense? 2. Which substance is least dense? 3. According to this table, are solids generally more or less dense than liquids? 4. What is more dense than aluminum but less dense than iron? 5. What substance has a density of 1 g/mL? 6. What substance has a density twice that of water? 7. List any two substances that have the same density. 8. Liquids on the list are those listed from water to Carbon Tetrachloride. List those five liquids from least dense to most dense. Density of an Irregularly Shaped Object (0.5 points) 9. With the help of the equipment shown in the picture, describe how you would determine the density of an irregularly shaped object. Densities of Some Common Materials Material Density (g/cm3) Aluminum Iron Wood (rock elm) Brass Cork Magnesium Paraffin Water Olive oil Alcohol Glycerin Carbon Tetrachloride Limestone Quartz Sulfur 2.7 7.8 0.8 8.5 0.2 1.7 0.9 1.0 0.9 0.8 1.3 1.6 2.9 2.6 2.0 Density(0.5 points each) Use the figure and your knowledge of mass, volume, and density to answer the following questions. 10. List objects A, B, and C in order from the object with the highest density to that with the lowest density. Explain how you determined your answer. 11. Object B is 2 cm long, 2 cm wide, and 2 cm high. What is its volume? 12. The liquid labeled C is water, which has a density of 1 g/cm3. There are 35 mL of water in the beaker. What is the total mass of the water in the beaker? 13. Describe a simple experiment you could use to determine if object D has a density that is less than or greater than water. 14. The volume of object A is 10 cm3. Its mass is 26 grams. What is the density of object A?