Density Powerpoint
advertisement

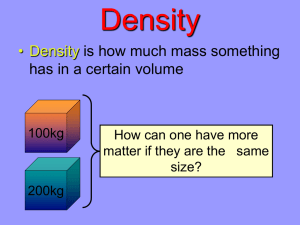
Density, Mass, & Volume Concepts & Equations Mass • Measurement of the amount of matter, or stuff, an object has – Measured in grams (g) Volume • Measurement of the amount of space an object takes up – Measured in milliliters (mL) or cm3 Density • Measurement of the amount of mass an object has per its volume – Derived unit: g/mL • Comes from units of mass per units of volume Density as a ratio • Density can be viewed as the ratio of an object’s mass to its volume Mass : Volume Graphing Density • On the vertical, or y-axis, mass is plotted • On the horizontal, or x-axis, volume is plotted • The resulting line that comes from plotting coordinates on the graph represents the density 1cc = 1mL Remember Slope? • Rise over run – It’s the amount that a y value changes compared to how much an x value changes • It’s a ratio of y values to x values Slope of Mass v Volume • If we figure out how much the mass changes (∆) compared to how much the volume changes, we get a slope that is a ratio of mass to volume – That slope will look like this: ∆Mass ∆Volume THAT’S DENSITY!!!!! The “bounceability” of a ball depends on its density, not on its mass or on how big the ball is. Drop height vs. Bounce height Density 200 Large ball – 0.53 g/cm3 B ounc e he ight (c m ) Medium ball – 1.05 g/cm3 Small ball – 0.82 g/cm3 101 grams 100 62 grams 19 grams 0 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 Drop height (cm) Density Equation • Density = mass divided by volume • g/mL = g divided by mL Let’s use density! D=m/v m d v How to use the circle m d v • Using info from problem, determine what you are trying to find. • Cover up the variable you’re trying to find • Do the calculation that’s left. EXAMPLE: Covering up mass leaves density x volume Problem 1 The mass of a silver bracelet is 2.5 grams, it occupies a volume of 48cm3, what is its density? Problem 2 The density of silver (Ag) is 10.5 g/cm3. Find the mass of Ag that occupies 965 cm3 of space. Problem 3 A 2.75 kg sample of a substance occupies a volume of 250.0 mL. What is its density? Problem 4 A rectangular block of Lead (Pb) measures 20.0 mm X 30.0 mm X 45.0 mm. If the mass of the Pb block is 10g, find its density. Problem 5 A cube of gold (Au), which has all equal sides, has a side length of 1.55 cm. If the sample is found to have a mass of 71.9 g, find the density of Au. Ways to Affect Density • Change mass AND keep volume same • Change volume AND keep mass same Change Mass AND Keep Volume Same • Increase the mass increase density • Decrease the mass decrease in density Which container has more density? A B Change Volume AND Keep Mass Same • Increase the volume decrease density • Decrease the volume increase density Which container has more density? A B What 2 ways will INCREASE density? What 2 ways will INCREASE density? Keep the same mass AND decrease the volume Keep the same volume AND increase the mass Comparing Densities • Objects or substances with MORE density will sink below objects or substances with LESS density – Which do you think is MORE dense, Water or Oil??? Water, Oil…and a Superball The oil is less dense than the water, so it’s on top. The superball is less dense than water, but more dense than oil, so it sinks to the bottom of the oil layer, yet floats on the top of the water layer. Why does ice float in water? Have you wondered why hot air balloons rise? When the air is heated, it becomes less dense until the balloon's total density is less than that of the atmosphere; A hot air balloon is literally floating on the denser, colder air. Why is cold air denser than hot air? Do you know why volcanoes erupt? The main reason that magma rises to the surface to erupt at volcanoes is because it is less dense than the rocks that surround it. A ship floating on water is a great illustration of the difference between mass and density. A ship must have a density of less than 1.0 g/cm3 (the density of water), or it will sink. Ships have a large mass, because they are made of steel, but because they have a large volume, their density is less than 1.0 g/cm3. If enough mass is added to them such that their density goes above 1.0 g/cm3, they will sink. In Conclusion • If you have 2 substances, – the MORE dense substance will be on bottom – The LESS dense substance will be on top Think About This • The density of five liquids are measured as follows: – Liquid 1: 1.0 g/mL – Liquid 2: 1.38 g/mL – Liquid 3: 0.77 g/mL – Liquid 4: 2.95 g/mL – Liquid 5: 0.056 g/mL • Draw a picture of all 5 liquids in a test tube how they would layer according to density Think About This • The density of five liquids are measured as follows: – Liquid 1: 1.0 g/mL – Liquid 2: 1.38 g/mL – Liquid 3: 0.77 g/mL – Liquid 4: 2.95 g/mL – Liquid 5: 0.056 g/mL Liquid 5 Liquid 3 Liquid 1 Liquid 2 Liquid 4 • Draw a picture of all 5 liquids in a test tube how they would layer according to density