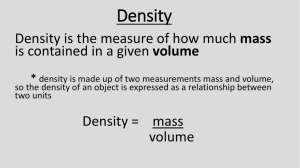STUDY GUIDE – Density
advertisement

STUDY GUIDE – Density Name: Date: Assess yourself on the following skills. Focus on your areas of weakness when you are preparing for the test! Learning Targets: I can do it! I can do with help! Totally Clueless! 1. Given the mass and volume of a substance, I can calculate its density. 2. Given the density and mass of a substance, I can calculate the volume. 3. Given the density and volume of a substance, I can calculate the mass. 4. I can explain what a characteristic property is, and how it can be used to help identify a substance. 5. I can explain why density is a characteristic property. 6. I can calculate the density of a substance by looking at a graph of its mass and volume. 7. I can predict the relative densities of liquids by observing how they form layers. 8. I can predict the relative densities of an object and a liquid by observing whether the object floats or sinks. VOCABULARY Density - _____________________________________________________________________________ Characteristic property - _________________________________________________________________ 1. A sample of rubbing alcohol has a volume of 0.50 cm3 and has a mass of 0.41 grams. What is the density of this sample of alcohol? Show your work. 2. A 0.50 cm3 block of rock salt has a mass of 1.07 grams. What is the density of this sample of rock salt? Show your work. 3. An 80.0 mL solution has a density of 5.80 g/cm3. What is its mass? Show your work. 4. What is the volume of 26.5 g of a solution whose density is 7.48 g/mL. Show your work. 5. You have two objects of equal volume. Object A has a greater density than object B. What can you say about the mass of object B? Write T for True or F for False. 6. ______ You can determine whether or not a sample of liquid is water if you know its volume. 7. ______ You can increase the density of an object by keeping the same mass but decreasing the volume. 8. ______ Volume can be measured in cm2 or mL. 9. ______ If the density of 50 mL of a liquid is 1.0 g/mL, then the density of 25 mL of the same liquid would be 0.5 g/mL. 10. Imagine that the liquids on the right have these densities: 15g/mL 3g/mL 7g/mL 10g/mL 9g/mL 12g/mL Label each layer with the correct density! a) The liquids shown above are separated due to differences in . b) What is the density of the most dense substance in the beaker? ______________ c) What is the density of the least dense substance in the beaker? ____________ 11. Study the graph above. Which substance has the greatest density? Explain. _____________________________________________________________________ 12. Can you calculate the slope of any of the lines?________ If you can, pick one and show work. 13. Using the same graph, could you determine if any of the densities represent water?If so, which one and why? 13. Do you understand how to use displacement to find volume?__________ What is the volume of the green jade? ___________ 15. If the green jade had a density 2 g/cm3, what would it’s mass be? Show work. 16. Can you identify an element using its density? _________ What element has a density of 1.8477 g/cm3? (Find in row 2 of table)___________________________ 17. Using the picture below, explain if one cylinder has a greater density than the other using the words mass and volume. 18. Explain how you could possibly prove that a gold colored pebble you found was truly gold. Explain all the steps as if you were actually doing it in the lab. 19. Explain why the density of an ice cube is less than that of liquid water. You may use pictures in your explanation.