DENSITY
advertisement

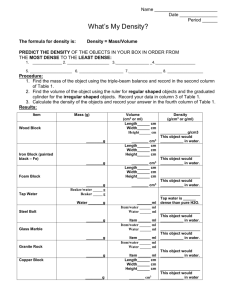
Density The amount of matter (mass) in a given space (volume) Solving for Density Density = mass divided by volume D = m/V If you have a glass of water with a mass of 500g (minus the glass), and the volume amounts to 500mL, what is the density of water? 500g/500mL = 1g/mL Density is a useful physical property for identifying substances, because each substance has a unique density. The density of a substance is always the same at a given temperature and pressure. Except…. ??? Density of Liquid Liquid density units are g/ml or kg/L When different liquids combine, the densest liquids form layers on the bottom, less dense layers form at the top. Water is the only non-metallic substance on Earth whose density in solid form (ice) is less than its density in liquid form. Why? Liquid water is most dense (1.00 g/mL) as a liquid and becomes less dense as the water molecules form crystals of ice as the temperature drops to 0 °C. This is due to hydrogen bonds forming between the water molecules, which line up molecules less efficiently (in terms of volume) when water is frozen. Hydrogen bonds are weak, constantly forming and breaking to create partially ordered structures in liquid water; in ice, each water molecule bonds rigidly to four others. Density of Solids Solid density units are g/cm3 or kg/m3 If you have a 1 kilogram of iron and a 1 kilogram of cotton, which would be less awkward to carry around? Since cotton is much less dense than iron, it would take up a lot more space, or volume. So it would be very awkward to carry a 1 kilogram of cotton (about 2.2 pounds). D = m/V Greater mass means greater density. Greater volume means less density. A solid floats when it is less dense than the liquid that its in, and sinks when it is more dense. So wood is less dense than water and a rock is more dense. Ice will suspend in water, most of its mass will be below water, and about 8%) will be above the water. This is because its density (.92 g/cm3) is very close the density of liquid water (1.0 g/mL) Buoyancy The upward force that a fluid exerts on an object less dense than itself. ~ Drews 2008 Measuring mass The amount of matter in an object. Use an electric balance to determine mass For solids use g, or kg For liquids, use the same method and units but remember to subtract the mass of the container from the total mass to find the mass of the liquid only. Example: 200g total mass – 40g beaker = 160g of liquid Measuring Volume The amount of space occupied by a 3 dimensional object. Volume of regular shaped solids can be determined using simple equations like =LxWxH For volume of a liquid, use graduated cylander. For irregular shaped solids, use the water displacement method. For solids, use cm3 or m3 For liquids, use mL or L Conversion: 1 cm3 solid = 1 mL liquid Density Calculation Practice What is the density of an object whose mass is 23.5 g and whose volume is 10 cm3? D = m/V D = 23.5g/10cm3 D = 2.35 g/cm3 Does the object float?