AP Chemistry Unit 1 Lab
advertisement
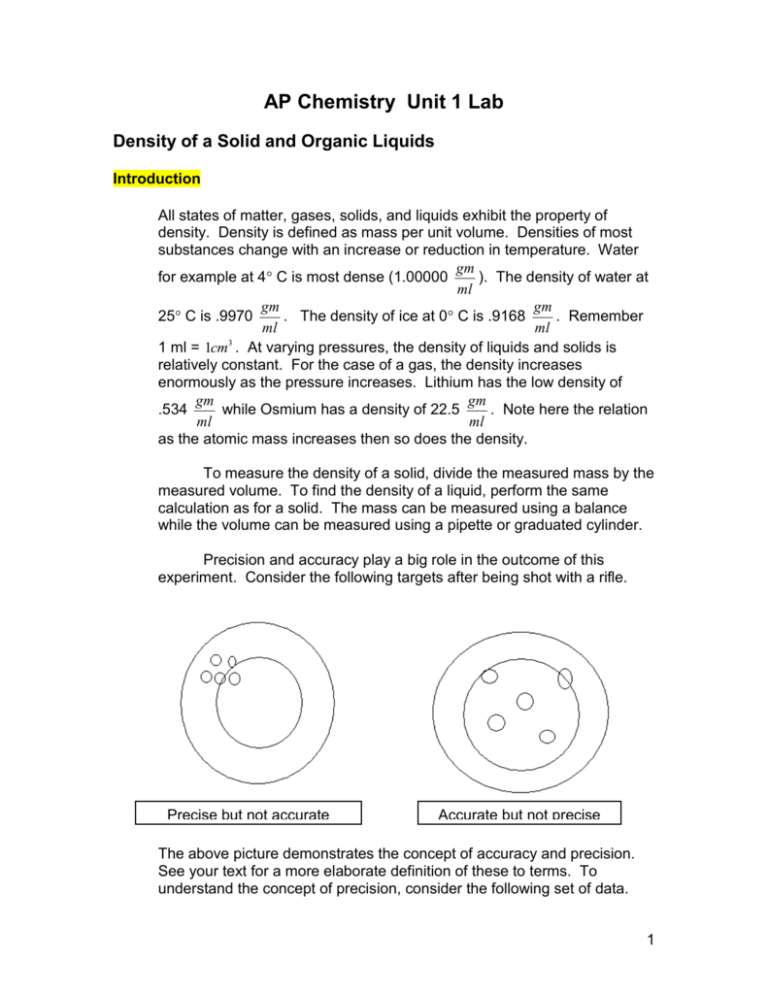
AP Chemistry Unit 1 Lab Density of a Solid and Organic Liquids Introduction All states of matter, gases, solids, and liquids exhibit the property of density. Density is defined as mass per unit volume. Densities of most substances change with an increase or reduction in temperature. Water gm for example at 4 C is most dense (1.00000 ). The density of water at ml gm gm 25 C is .9970 . The density of ice at 0 C is .9168 . Remember ml ml 1 ml = 1cm3 . At varying pressures, the density of liquids and solids is relatively constant. For the case of a gas, the density increases enormously as the pressure increases. Lithium has the low density of gm gm .534 while Osmium has a density of 22.5 . Note here the relation ml ml as the atomic mass increases then so does the density. To measure the density of a solid, divide the measured mass by the measured volume. To find the density of a liquid, perform the same calculation as for a solid. The mass can be measured using a balance while the volume can be measured using a pipette or graduated cylinder. Precision and accuracy play a big role in the outcome of this experiment. Consider the following targets after being shot with a rifle. Precise but not accurate Accurate but not precise The above picture demonstrates the concept of accuracy and precision. See your text for a more elaborate definition of these to terms. To understand the concept of precision, consider the following set of data. 1 Experiment 1 2 3 4 5 Measured Density 0.98 gm/ml 0.96 gm/ml 0.99 gm/ml 0.95 gm/ml 0.97 gm/ml Average = 4.85/5 = 0.97 Absolute Deviation 0.01 0.01 0.02 0.02 0.00 Average Deviation = 0.06/5 = 0.01 gm 0.01. Disregard any negative ml sign in the calculation of deviation or average deviation. So then we can say the density is 0.97 Table of Known Densities – gm/ml Pentane ............................................................. 0.626 Hexane .............................................................. 0.659 tert-Butyl Methyl Ether ....................................... 0.741 Toluene ............................................................. 0.867 Ethyl alcohol ...................................................... 0.785 Ethyl Acetate ..................................................... 0.902 Acetone ............................................................. 0.791 Carbon Tetrachloride ........................................ 1.594 Dichloromethane ............................................... 1.325 Materials for this lab Milligram Balance 1 ml Syringe – graduated a “TC” or “TD” pipette 2 sample vials and caps 2 cm length of rubber tubing Graduated Cylinder Forceps Safety Considerations Ware safety goggles Ware a lab apron All reagents used in this experiment are combustible. Do not use fire near them. 2 Pre Lab Questions 1. In the introduction of this experiment, how many significant figures is the density of water given at 4 C and at 25C? 2. A one-liter flask is placed on a one pan of a very accurate balance. On the other pan brass weights are placed such that both pans balance exactly. This entire apparatus is placed in a complete vacuum. The balance is tipped in favor of the flask. Is seems to weigh more. Explain why. 3. Determine the relative error if the experimental density is gm gm measured at: .735 and the actual density is .750 . ml ml 4. What does average deviation of a set of data tell us? Procedure Solid 1. 2. 3. Liquids 1. 2. 3. 4. Measure the mass of the unknown solid and record in the report sheet. Pour a specified volume of water in a Graduated cylinder. Use a cylinder large enough so that the metal object will easily pass into it. Insert the metal object and record the volume change. Examine the pipette carefully. Two types are most commonly used. One will be labeled “TC” at the top (to contain) and “TD” at the bottom (to deliver). The “TD” pipette delivers 1.00 ml from the top graduation to the bottom of the pipette whereas the “TC” pipette delivers 1.00 ml from the top graduation to the bottom graduation marked “0”. Either pipette will work. Be sure to familiarize yourself with use of the pipette. Attach a 1 ml syringe to the pipette using a 2 inch section of a gum rubber tube. Determine the masses of the solid metal object supplied by the teacher and 2 labeled and caped vials. Be sure your measure the masses to the nearest .001 gms. Using the syringe as a pump, draw liquid into the pipette. Try to get the liquid near to the top graduation. Record the amount of liquid in the pipette within .001 ml. The last figure will be an estimate. Expel a portion of the liquid from the pipette into the vial. Again record the volume reading from the pipette. Determine the masses of the liquids and vials to within .001 3 Report Sheet Unknown solid Mass of Solid __________________ Final Volume of graduated cylinder __________________ Initial volume of the graduated cylinder __________________ Volume of the solid __________________ Known Liquid Name of known liquid _______________________ Mass of empty Graduated Cylinder ____________ Mass of Graduated Cylinder and liquid ____________ Mass of liquid ____________ Volume of liquid ____________ Known Liquid Name of known liquid _______________________ Mass of empty Graduated Cylinder ____________ Mass of Graduated Cylinder and liquid ____________ Mass of liquid ____________ Volume of liquid ____________ Calculations Calculate the density of the solid Compare your calculated density to densities of known solids using the CRC handbook. Using the stated density in the CRC handbook perform an error analysis. Calculate the density of the liquid 1 Using the table provided in this experiment, calculate an error analysis. Calculate the density of the liquid 2 4 Using the table provided in this experiment, calculate an error analysis. Post Lab Questions 1. Explain why there was error in your experiment. 2. Most of your error was probably in measuring the volume used in the calculation of density for the solid. What way could be used to measure the volume of the solid? 3. Can the determination of density be used to identify a material? Why or why not? 4. For the case of the rifle targets shown in the introduction, what would the target look like with high accuracy and high precision? Explain. 5









