Fundamentals of Chemistry 1030
advertisement

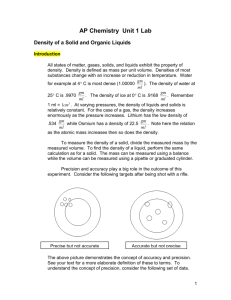
Fundamentals of Chemistry 1030 Measurements I WEAR SAFETY GLASSES in LAB Background Information (or Introduction) Chemistry is often called the central science. Chemistry is also an experimental science in which its knowledge base has been established by millions of experiments. In these experiments a vast wealth of data has been collected. Much of the data has come from measuring. Fundamental to chemistry is the understanding and ability to measure. All measurements have three characteristics: (1) a numerical value, (2) a unit and (3) a degree of uncertainty. Measurements are never exact and have ranges of precision and accuracy dependent upon the instrument used and human ability. This experiment focuses on mass and volume measurements. Chemistry is defined as the study of matter, its properties, and how energy can change matter. Matter can be defined as having mass and occupying space. The relationship between mass and volume (space occupied) leads to one of the first physical properties studied in chemistry or other physical science: density. Mathematically, density is determined by dividing the mass of an object by its volume. Density mass volume or D m V In lab you will measure mass by using the electronic balance and determine volume by using a meter stick (ruler), graduated cylinder, or graduated pipette. Mass will be recorded in the unit of grams (to the nearest 0.001 g) and volume will be recorded in the unit mL (to the nearest 0.1 mL). Density will then be determined and presented as g/mL. Come to lab prepared by having a data table developed in your lab report. Read the following procedure carefully (maybe several times) to determine what data you will collect and in order for you to determine how to organize the table. Part I Procedures for Determining Density of a Solid A. Determining the Mass of Solid 1. Demonstration: Your lab instructor will demonstrate the proper use of the laboratory balance and other equipment. 2. Mass of Solid: Determine the mass of each solid object using the balance (record to the nearest 0.001g). In CHEM 1030 labs all mass determinations will be recorded to the nearest 0.001 gram. B. Measuring Volume of Solid. This will be done by using two methods: direct and displacement. Make sure you note in your data table which volume determination is direct and which is displacement. You will do both methods for each of the two solid samples. 1. Volume of Solid - Direct Measurement. Obtain 2 solid samples from your instructor. a) Record the sample# . b) Record its shape. c) Use the meter stick (or ruler) to determine the dimensions of the solid in milliimeters. Since the equation for determining volume depends on the shape of the object, use the chart below to determine which dimensions are needed to determine the volume of your solid object. For example, if the object is a cylinder, only height and diameter are needed. d) Use the table below to calculate the volume of the solid. In using these formulas convert mm lengths to cm lengths (10 mm = 1 cm), This will provide all volumes in cm3 Shape Dimensions Volume (cm3) cube length (L) V = L3 rectangular length (L), width (W), height (H) V=LxWxH cylinder diameter (d), height (H) V = (3.14 x d2 x H) /4 2. Volume of a Solid by Volume Displacement. When a solid object is submerged under water; it displaces the water by the amount of its own volume. The difference in the water level before and after the object is submerged is equal to the volume of the object submerged. a) Obtain a 25.0-mL graduated cylinder and fill it with water until it is about half full. Record the volume of the water to the nearest 0.1 mL. Hold the graduated cylinder at an angle of approximately 45° and slowlySlowly and carefully slide the object into it. Be careful not to splash any drops of water on the sides of the cylinder. Record the new volume of the water to the nearest 0.1 mL. b. Convert the volume of the solid from milliliters to cubic centimeters (1 mL = 1 cm 3) C. Determining the Density of Solid Density is defined as mass in grams per unit volume in milliliters or g/mL. Density of solid = Mass (g) of solid/Volume (mL) of solid Part II Procedures for Determining Density of a liquid A. Determining the Mass and Volume of a Liquid. 1. Determine the mass of liquid and its volume: Obtain a dry 100-mL beaker and record its mass to the nearest 0.001 g. Using a 10.00-mL graduated pipette and pipette pump, acquire 10.00 mL of an unknown liquid. Be sure to record which unknown liquid you select. (Your instructor will demonstrate how to use the graduated pipette and pump). (Use care as glass pipettes can easily break if used improperly). Deliver 10.00 mL of solution into the pre-weighed 100-mL beaker. Record its mass. Calculate the mass of the unknown liquid by subtracting the mass of the beaker from the mass of the beaker plus solution. 2. The mass difference is the mass of the liquid. The volume of the liquid ()should be10.00 mL, provided you used the pipette correctly. Calculate the density of the unknown liquid using the density formula. CALCULATIONS AND ANALYSIS: Show all calculations below your Data Table in the Lab Report 1. Volume from Direct Measurement 2. Volume from Displacement Measurement 3. Density of Unknown Solid (include sample #) 4. Identify the Unknown Solid (include sample #) by comparing the density calculated for the Unknown with the densities of known materials provided by the instructor. 5. Mass of the Unknown liquid 6. Density of the Unknown liquid (include sample #) Questions to be addressed in your conclusion: How close are the results for the volume of the solid from measuring the dimensions to the volume you determined by displacement of water? Which method of volume measurement do you think is most accurate: direct measurement or measurement by displacement? Why? Why is a beaker not the appropriate device to measure volumes of liquids? And what are the purposes and use of beakers as laboratory equipment? Is it possible to determine the mass of an object if you know only its volume and density? Explain your answer. It is possible to determine the volume of an object if you know only its mass and density? Explain your answer.