Mass, Volume, and Density Worksheet
advertisement
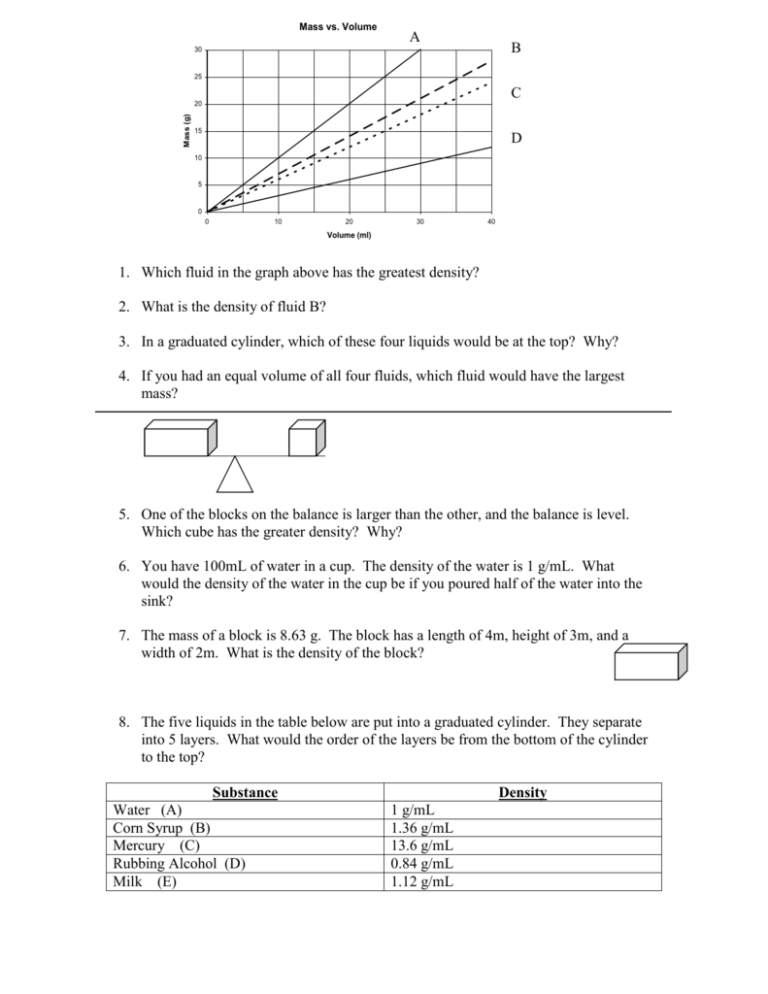
Mass vs. Volume A B 30 Substance 1 25 Substance 2 C Mass (g) 20 15 D Substance 3 10 5 0 0 10 20 30 40 Volume (ml) 1. Which fluid in the graph above has the greatest density? 2. What is the density of fluid B? 3. In a graduated cylinder, which of these four liquids would be at the top? Why? 4. If you had an equal volume of all four fluids, which fluid would have the largest mass? 5. One of the blocks on the balance is larger than the other, and the balance is level. Which cube has the greater density? Why? 6. You have 100mL of water in a cup. The density of the water is 1 g/mL. What would the density of the water in the cup be if you poured half of the water into the sink? 7. The mass of a block is 8.63 g. The block has a length of 4m, height of 3m, and a width of 2m. What is the density of the block? 8. The five liquids in the table below are put into a graduated cylinder. They separate into 5 layers. What would the order of the layers be from the bottom of the cylinder to the top? Substance Water (A) Corn Syrup (B) Mercury (C) Rubbing Alcohol (D) Milk (E) Density 1 g/mL 1.36 g/mL 13.6 g/mL 0.84 g/mL 1.12 g/mL A B C 9. Rank above in order of least energy to most energy. 10. Rank above in order of least dense to the most dense? 11. Which product best represents possibly a solid. Why? 12. Which product above best represents possibly a gas. Why? 13. What is the density of a block with a mass of 8.2 g and a volume of 14mL? Will it float on water? Why? 14. What is the mass of a block with a density of 13.2 g/cm3 and a volume of 5.2 cm3? Will it float on water? Why? 15. A. A graduated cylinder is filled with 22mL of water. A 25.0 g marble is dropped into the water, and the water level rises to 29mL. What is the density of the marble? 16. Describe buoyancy and how it is related to density. 17. Rank the five substances for highest viscosity to lowest viscosity. Substance Water (A) Corn Syrup (B) Mercury (C) Rubbing Alcohol (D) Milk (E) Rate of Flow 3.6 cm/s 1.6 cm/s 4.3 cm/s 4.0 cm/s 2.3 cm/s











