Lab Report: Dehydration of an Alcohol (Exp
advertisement
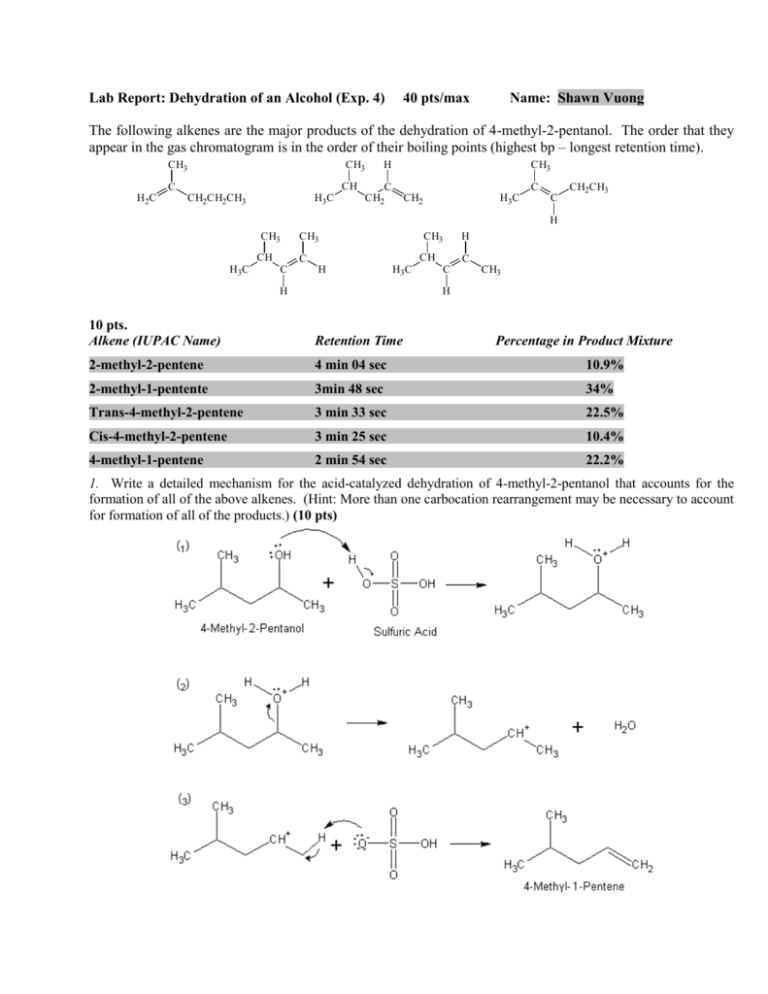
Lab Report: Dehydration of an Alcohol (Exp. 4) 40 pts/max Name: Shawn Vuong The following alkenes are the major products of the dehydration of 4-methyl-2-pentanol. The order that they appear in the gas chromatogram is in the order of their boiling points (highest bp – longest retention time). CH3 CH3 CH C H2 C CH2CH2CH3 H3 C H CH3 C CH2 C CH2 H3 C C CH2CH3 H CH3 CH H3 C CH3 CH3 CH C C H H3 C H H C C CH3 H 10 pts. Alkene (IUPAC Name) Retention Time 2-methyl-2-pentene 4 min 04 sec 10.9% 2-methyl-1-pentente 3min 48 sec 34% Trans-4-methyl-2-pentene 3 min 33 sec 22.5% Cis-4-methyl-2-pentene 3 min 25 sec 10.4% 4-methyl-1-pentene 2 min 54 sec 22.2% Percentage in Product Mixture 1. Write a detailed mechanism for the acid-catalyzed dehydration of 4-methyl-2-pentanol that accounts for the formation of all of the above alkenes. (Hint: More than one carbocation rearrangement may be necessary to account for formation of all of the products.) (10 pts) 2. Why must the distillation temperature be kept below 90 oC during the dehydration of 4-methyl-2-pentanol? (5 pts) If the temperature would raise above 90 degrees Celsius the potential for water evaporation would increase. Therefore, water could get into the product and defeat the purpose of the dehydration. 3. Which conclusions can be drawn from the IR-spectrum of the reaction mixture? (5 points) According to the IR spectrum, there are molecules of molecular weight of 84 grams in the mixture. *All alkenes products of 4-methyl-2-pentanol have a molecular weight of 84 grams. 4. Predict all of the alkene products that are expected (including stereoisomers and alkenes arising via carbocation rearrangements) from dehydration of the following alcohols. Indicate which of these products should be the major product of the reaction. (5 points) a. 3-methyl-2-butanol b. 2-methylcyclopentanol c. 3-cyclohexen-1-ol OH HO CH3 H3C CH3 H3C OH __________________________________________________________________________ 5. Open the UCAN program. Which process is depicted by the first curved arrow in the E1 animation? (3 pts). Lewis Acid Base Dissociation *to produce a carbocation intermediate Open the ORA program. How many reaction steps are shown on the animation “Carbocation rearrangement:E1”? (2 pts). There are four steps