Problem Set 2
advertisement
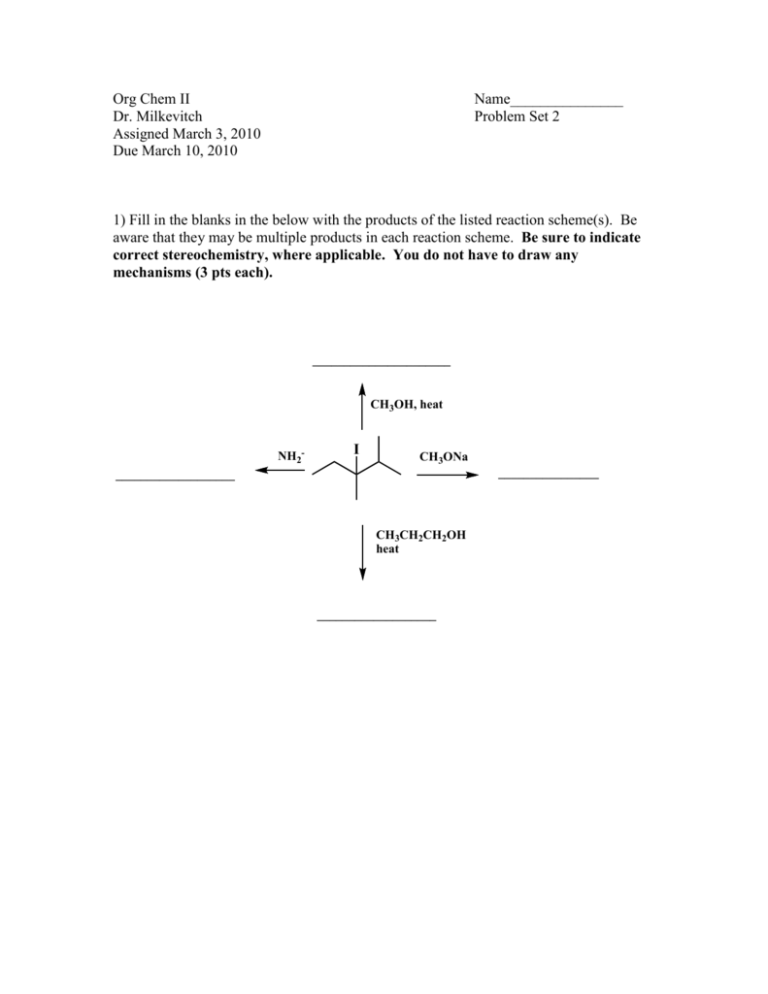
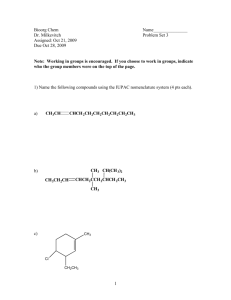
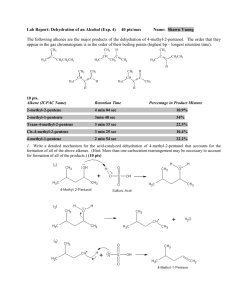
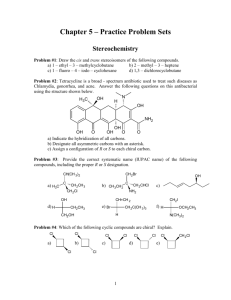
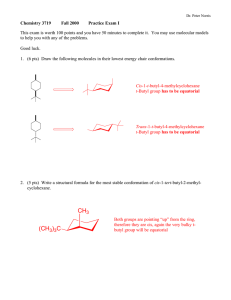
Org Chem II Dr. Milkevitch Assigned March 3, 2010 Due March 10, 2010 Name_______________ Problem Set 2 1) Fill in the blanks in the below with the products of the listed reaction scheme(s). Be aware that they may be multiple products in each reaction scheme. Be sure to indicate correct stereochemistry, where applicable. You do not have to draw any mechanisms (3 pts each). ______________________ CH3OH, heat NH2- I CH3ONa ________________ ___________________ CH3CH2CH2OH heat ___________________ 2) When 2-bromo-2-methylpropane is dissolved in aqueous methanol at 25C, a mixture of the below products is obtained. Draw mechanisms to account for each of the products formed (4 pts each mechanism). CH3 H3C C OCH3 CH3 CH3 H3C C OH CH3 H3C C H3C CH2 3) Predict the structure of the all the alkene products formed in the E2 reactions of the following halides (3 pts each). a) Br b) Cl c) I 4) Predict all substitution and elimination products of the following reactions (4 pts each). a) Br H2O b) Br c) H2O CH3OH CH2Br 5) Provide the IUPAC names for the following alkenes (3 pts each). a) b) c) H3CH2C F e) I H3CH 2CH 2C CH 2CH 3 C H2C g) F d) CH3 CH CH3 C f) CH 3 h) CH 3 CH 2CH 3 F I 6) Examine the structure of the following compounds and determine if they can be named using cis-trans nomenclature or E-Z nomenclature. Draw and label the isomers, using cis-trans or E-Z nomenclature, where applicable. If you find that cis-trans nomenclature or the E-Z nomenclature system is not applicable, then name the alkene as normal (4 pts each). a) 2-pentene b) 1,1-dicloropropene c) hexa-2,4-diene 7) Predict the products of the following reactions. If more than one product is possible, predict which will be the major product. You do not have to write mechanisms (3 pts each). a) OH H2SO4, heat b) H3PO4 , Heat OH c) 1-bromo-1-methylcyclohexane + triethylamine d) + K+ t-butox (CH3)3COH Cl e) I + f) OH H2SO4 heat (CH3)3COK (CH3)3COH