Acids, Bases, and Indicators: A Colorful Activity
advertisement

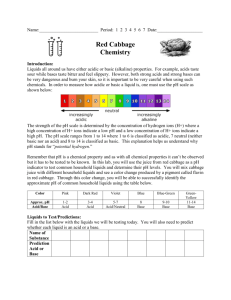
WISE 2007-2008 Name: ____________________________________ Acids, Bases, and Indicators: A Colorful Activity! Purpose: To label common household solutions as acidic or basic using cabbage juice and litmus paper. Introduction: Water molecules collide and produce H+ and OH- ions from the H2O compound. The hydrogen ion, H+, is characteristic of an acidic solution, while the hydroxide ion, OH-, is characteristic of a basic solution. Water is a neutral substance because the concentration of the two ions is equal. As solutes are added to water to make solutions, however, the balance of H+ and OH- is changed. + H+ + OH- H2O A measure of the hydrogen ion (H+) concentration is pH. Low values of pH indicate an acidic solution (lots of H+). High values of pH indicate a basic solution (lots of OH-). Acids have a pH less than 7, bases (or “alkaline” substances) have a pH greater than 7, and neutrals have a pH of 7. Example of a pH scale Each whole pH value below 7 is ten times more acidic than the next higher value! For example, a pH of 3 is ten times more acidic than a pH of 4. For pH values above 7, each whole value is ten times more basic than the next lower value! Acids taste sour while bases taste bitter and are slippery. Changing the hydrogen ion concentration will cause some natural dies to change colors. Just as the color of tea will change as lemon is added, red cabbage juice will have a different color in acids and bases. Dyes which change color in response to the change of pH are called acid-base indicators. WISE 2007-2008 Name: ____________________________________ Experiment: The lab activity today will explore the use of red cabbage juice as a natural acid-base indicator. In the test tube rack you will find an example of an acid, water (a neutral substance), and basic solution with the cabbage juice added. What is the color of cabbage juice in an acid? _____________________________________________ What is the color of cabbage juice in a base? ______________________________________________ What is the color of cabbage juice in water? _______________________________________________ Now put 10 drops of your first unknown sample into one well of the dropping tray and add 2 drops of cabbage juice extract. Repeat this procedure with each sample. Sample Color With Cabbage Juice Acid/Base/Neutral Liquid soap Sprite soda Vinegar Baking soda Lemon juice Sugar solution Windex Extension activity: Put your name on a piece of white paper on the table. Now using a Q-tip, dip into the solutions and make an invisible design on your paper. Vary the acids and bases used on the paper in order to create different colors. When the solutions have been absorbed, the paper will be sprayed with cabbage juice to reveal your design!