A pH Exercise with Red Cabbage Juice REVISED
advertisement

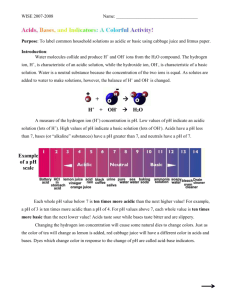
Experiment 14: Measuring Acidity with Red Cabbage Juice Many common household chemicals can be described as acids or bases. For example, many cleaning supplies are basic (sometimes called alkaline). Vinegar is a 5% solution of acetic acid (the remainder is water). Shampoos and cosmetic products are usually pH balanced to a level which minimizes irritation of the skin. In this experiment, you will test various substances and designate them as an acid, a base, or neither (neutral). Acids are substances that can donate a hydrogen ion in water; bases are substances that can accept a hydrogen ion or produce hydroxide ion in water. The amount of hydrogen ion donated or accepted determines the strength of the acid or base. The concentration of hydrogen ion in a solution is described by its pH value. The pH of a solution is mathematically defined as pH log[ H ] where, [H+] is the concentration of the hydrogen ion (i.e. the amount of hydrogen ion per liter). The negative logarithmic relationship means that every time the pH increases by 1, the hydrogen ion concentration decreases by a factor of 10. A lower pH value corresponds to a higher acidity of the solution. A Logarithms & Earthquakes pH above 7 is basic, and pH below 7 is acidic. For a neutral solution, the pH is 7. Many naturally The Richter Scale measures the relative strengths occurring molecules are influenced by the level of of earthquakes. Like pH, the Richter Scale is a the acidity around them. The hydrangea flower has logarithmic scale. For example, an earthquake measuring 6.0 is ten times stronger than one that a red color in acidic soil, but blue in basic soil. measures 5.0; similarly, an earthquake that Molecules that change color in response to changes measures 5.0 is ten times stronger than one in acidity level are called indicators. Some which measures 4.0. The negative sign in the pH indicators change color only at one particular pH, formula reverses the effect, so a solution with pH whereas others exhibit numerous numbers of colors 4.0 is ten times less acidic than one with pH 3.0. over a broad pH range. Red cabbage leaves contain Logarithmic relationships are more complex than a compound that exhibits different colors at you might expect. For example, an earthquake different pH values. In this experiment, you will measuring 4.5 is not five times stronger than one measuring 4.0, as many people might assume (in examine the color changes which accompany the fact it is only 3.16 times larger). pH changes. Procedure: Note that your instructor may recommend that you work in pairs for some or all of this experiment. A. Determine the indicator’s color at various pH values 1. Obtain about 5 large red cabbage leaves and tear them into small pieces. Place them in a 400mL beaker and cover them with DI water. 2. Heat the cabbage water until the water boils. Boil it for 5 to 7 minutes, then turn off the burner. 3. Pour the cabbage juice into a 250-mL beaker and discard the leaves. Allow the juice to cool to room temperature. 4. Obtain 12 clean test tubes and a test tube rack. Label the test tubes from 1 to 12 to correspond with the pH values of the solutions you will be testing. Page 14-1 5. Add about 2 mL of the cabbage juice to each of the test tubes. Then, add about 2mL of the standard solution labeled pH=1 to test tube 1, and pH=2 to test tube 2, and so on. Think of a way to do this step without measuring the volume of solutions repeatedly. 6. Record the colors of the solutions in the test tubes in the data table on the report sheet Save these test tubes to use as standards for comparison to the household substances you will be testing in Part B. B. Determining the pH values of household substances 1. Obtain 10 additional test tubes and label them to indicate which household substance is in each. 2. Add 2mL of cabbage juice to each of the 10 test tubes. 3. If the substance is a liquid, add 2ml of it to a test tube. If the substance is viscous (i.e. syrupy, like baby oil), add only few drops to a test tube and use a stirring rod to swirl the substance in the test tube. If the substance is a solid, add a small spatula tip of it (about the size of a pea) to the test tube containing the cabbage juice. 4. Record the color of each household substance with cabbage juice on the report sheet. Compare these solutions to the standard solutions from part A and estimate the pH based on the color. 5. Categorize your household substances as acids, bases, or neutral. Page 14-2 Report Sheet Name:___________________________ Measuring Acidity with Red Cabbage Juice A. Determine Indicator’s Color at Various pH pH 1 2 3 4 5 6 Color pH 7 8 9 10 11 12 Color B. pH of Household substances substance Color in Cabbage Juice pH Acid. Base or Neutral? Vinegar Ammonia Lemon Juice Apple Juice 7-UP Ivory Liquid Detergent Shampoo Hair Conditioner Antacid Aspirin Mouthwash Windex Wisk Cleaners Ajax Corn Syrup Page 14-3 Questions Suppose that you have two samples of vinegar, although each contains a different volume of it. Sample A contains 100 mL, while Sample B contains 500 mL. Other than the difference in volume, the vinegar sample are identical. Is one sample “more acidic” than the other? If so, then identify the more acidic one. Give a brief explanation of your answer. Five drops of an indicator is added to each of the samples in the previous question, and both solutions turn orange. The color in Sample B is fainter than that of Sample A, although the actual shade of the color is the same. Explain these two observations. Does the information given in the second question seem to be consistent with what you would expect from your answer to the first question? Explain briefly. Page 14-4 Materials for this experiment: Please refer to chem 110 lab -We will use the same standard buffers and the same household good for this experiment. Cabbage We will not use pH paper nor pH meters. Page 14-5