Mystery pH Lab
advertisement

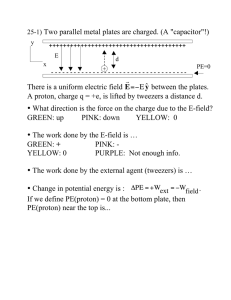
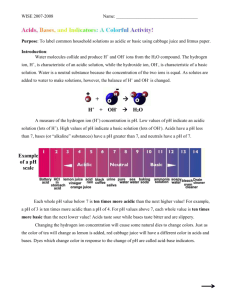
Mystery pH Lab Problem: Find the pH of different solutions using acid/base indicators. Materials: Two 24-well plates, 6 different pH indicator solutions, solutions of unknown pH, purple cabbage juice (for part II) Instructions: Pipette just enough of the unknown solution into a test well to cover the bottom of the well. Add one drop of indicator solution. Make a chart to record data. Use the information below to figure out the pH of the unknown solution to within as narrow a range as possible. Solutions of Unknown pH .1 M HCl (Hydrochloric acid) .1 M NH4OH (Ammonium Hydroxide) Boric Acid .1 M NaOH (Sodium Hydroxide) Vinegar Indicators Alizarin Yellow- Dark red at pH 12 or above Phenolphthalein- Colorless below 8.2. Dark pink above 10. Between 8.2 and 10 it shades from very light pink to dark pink. Bromothymol Blue- Yellow below 6. Blue above 7.6. Shades from yellow-green to green-blue from 6 to 7.6. Methyl Orange- pinkish-red or orange-red below a3.1. Yellow/orange above 4.4. Methyl Violet- yellow below 0.1 and violet above 3.1. Shades from yellow-green to blue between .1 and 3.1. Orange IV- dark pink below1.4, and yellow above 2.6. Yellowish-pink if between 1.4 and 2.6 Part II After you have discovered the approximate pH of the unknown solutions, use those solutions and some purple cabbage juice to write a description of the color changes for purple cabbage juice as the pH increases from low to high. Clean Up: Make sure all lids are snapped down on bottles. Rinse 24-well plates thoroughly.