Red Cabbage Chemistry
advertisement

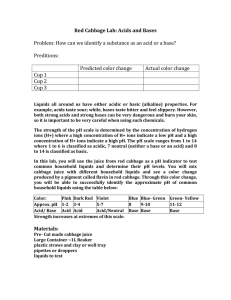
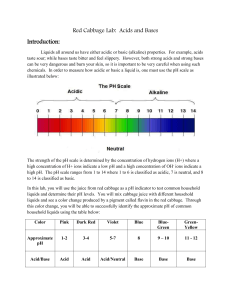
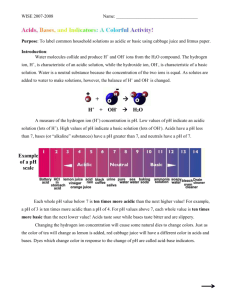
Name:__________________________ Period: 1 2 3 4 5 6 7 Date:____________________ Red Cabbage Chemistry Introduction: Liquids all around us have either acidic or basic (alkaline) properties. For example, acids taste sour while bases taste bitter and feel slippery. However, both strong acids and strong bases can be very dangerous and burn your skin, so it is important to be very careful when using such chemicals. In order to measure how acidic or basic a liquid is, one must use the pH scale as shown below: The strength of the pH scale is determined by the concentration of hydrogen ions (H+) where a high concentration of H+ ions indicate a low pH and a low concentration of H+ ions indicate a high pH. The pH scale ranges from 1 to 14 where 1 to 6 is classified as acidic, 7 neutral (neither basic nor an acid) and 8 to 14 is classified as basic. This explanation helps us understand why pH stands for “potential hydrogen.” Remember that pH is a chemical property and as with all chemical properties it can’t be observed but it has to be tested to be known. In this lab, you will use the juice from red cabbage as a pH indicator to test common household liquids and determine their pH levels. You will mix cabbage juice with different household liquids and see a color change produced by a pigment called flavin in red cabbage. Through this color change, you will be able to successfully identify the approximate pH of common household liquids using the table below. Color Pink Dark Red Violet Blue Blue-Green Approx. pH Acid/Base 1-2 Acid 3-4 Acid 5-7 Acid/Neutral 8 Base 9-10 Base GreenYellow 11-14 Base Liquids to Test/Predictions: Fill in the list below with the liquids we will be testing today. You will also need to predict whether each liquid is an acid or a base. Name of Substance Prediction Acid or Base Lab Instructions: 1. You will need approximately 25mL of red cabbage juice for each of the 8 liquids that you will be testing. This means that you will need 200mL of cabbage juice for all 8 of your tests. 2. For each liquid that you are testing you will take 50 mL of the liquid that you are testing and add it to 25mL of the cabbage juice indicator. You will need to stir each sample thoroughly to make sure that the two liquids mix. 3. There might be some solids that your teacher wants you to test so you will need to mix these solids into water before testing their pH. (For example you might test out baking soda by adding three tablespoons of baking soda to 25mL of water) 4. Please leave out each sample so that you can compare them to one another and estimate their overall pH level. After you have estimated their pH levels put them in order from most acidic to most basic like an actual pH scale. Experiment Results: Name of Substance Color Change Predicted pH Actual pH Real Life Applications: 1. Neutralization occurs when you mix an acid with a base, they neutralize each other. If this is the case, why is Alka-Seltzer used to treat stomach aches? (Excess stomach acids cause stomach aches) 2. What is acid rain, how does it form, and how does it negatively impact environments such as oceans, rivers, lakes, and ponds?