Fundamental Concepts of Thermodynamics
advertisement

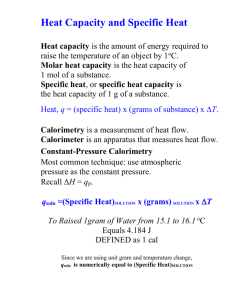
Thermodynamics • Is a policeman • Eliminates the impossible • Identifies the improbable • Simplifies your life – Links what can be measured to what you really want to know – Limits the number of independent variables • Is a macroscopic formalism that links to microscopic insights – Quantum mechanics statistical mechanics thermodynamics – Links directly to structure and dynamics in solids Fundamental Concepts of Thermodynamics • • • • • • • First, second, and third law Entropy Heat capacity, enthalpy Reaction enthalpies and thermochemical cycles Phase transitions Calorimetry Pressure as a variable Why I Count Calories for a Living • They are fascinating – Energetics whisper secrets of the strength of chemical bonds – Entropies sing of vibrating atoms, moving electrons, and structural disorder – Systematics have predictive power • They pay – Thermodynamic data are essential to good materials processing – Environmental science needs thermodynamics, both for issues of stability and as a starting point for kinetics – Mineralogy, petrology, and deep Earth geophysics need thermodynamic data. From thermochemical data one calculates • Entropies and free energies • Solubilities • Phase diagrams • Petrologic and geochemical processes • Materials synthesis and compatibility What is a Phase Transition? • A change in structure usually accompanied by a change in symmetry • May occur at a single temperature or spread over a temperature range • May be reversible and non-quenchable, reversible and quenchable, reversible with hysteresis, or irreversible • May involve positional, magnetic, or electronic disordering Phase Transitions Example – Heat Capacity and Entropy of Fe2SiO4 Olivine and Spinel Polymorphs Navrotsky et al. Proc. Natl. Acad. Sci., 104, 9187-9191 (2007) Ideal Cubic Perovskite ABO3 Distortions in Perovskites: octahedral tilting Why Distortions? • Ideal cubic structure defines bond distance in 12-coordinated A-site and 6-coordinated B-site simultaneously • Many A-site ions are “too small” and prefer lower coordination • Octahedra tilt, also sometimes B-cations offcenter ===> a whole family of related structures of varying symmetry, often with only very small distortions • Increasing temperature leads to phase transitions to higher symmetry structures Structural Studies: Xrays vs. Neutrons Xrays • Lab Xrays easiest but of limited resolution • Synchrotron Xrays - higher intensity, resolution Neutrons • Detect light elements (e.g. H,D), distinguish elements with similar atomic number • Accurate crystallographic parameters, see small changes in symmetry • See magnetic transitions and ordering • Obtain vibrational density of states and use to calculate entropy Whole pattern fitting - Rietveld methods In situ studies CaTiO3 Perovskite Transitions • In situ neutron diffraction and Rietveld refinement at 296-1729 K and heat capacity measurements • Orthorhombic (Pbnm) – tetragonal • (I4/mcm) reversible transition at 1513 +13 K and tetragonal (I4/mcm) – cubic (Fm3m) reversible transition at 1635 +2 K CaTiO3: Axial Ratios by in situ Neutron Diffraction M. Yashima and Y. Ali, Solid State Ionics 180, 120-126 (2009) CaTiO3: Heat Capacity P. Guyot et al. Phys. Chem. Min. 20, 141-146 (1993) Thermal Analysis and Scanning Calorimetry • Measure a signal (mass, heat, evolved gas, lemgth, X-ray pattern) at a variable heating (cooing) rate • Systems – Room temp to 600 oC, common – 600-1500 oC, less common but we have – 1500-2400 oC, uncommon but we have Calorimetry above 1500 oC Example: HfO2 Unit cell volume, Å 3 Mon (P21/c) 144 Cub (Fm3m) Tetr (P42/nmc) ZrO2 HfO2 140 J. Wang et al. J. Mat. Sci. 92, 27(20) 5397 400 800 1200 1600 2000 Temperature, °C Experiment: HfO2_calibr_probe_3rd_heating Displacement/µm SETSYS Evolution - 2400 Probe: Graphite Carrier gas: Length (mm): 2.502 mm Procedure: 1500>2300 (Zone 1) 0.0 TMA on HfO2 Heating -5.0 -10.0 Delta : -0.71 % Delta : 1.01 % -15.0 -20.0 Cooling -25.0 1800 1850 1900 Furnace temperature /°C TMA traces of HfO2 (1.5 % Zr) in Ar flow. (HfO2 pellet L 2.5 mm Ø 5 mm sintered at 1700 °C for 2 hours). Heating rate 10 °C/min. 5 gram load. \ SETSYS Evolution - 2400 Experiment: 3rd_HfO2_W_10_1950 Crucible:W 85 µl Carrier gas: Procedure: 20>2000 (Zone 3) Molar mass: 210.49 Mass (mg): 205.71 DTA signal, µV -bl_cooling - 4 - 3r d_HfO2_W_10_1950/µV -bl 2/µV Exo 2 2.5 Exo 1 1.5 Peak :1762.13 °C Onset Point :1771.45 °C Enthalpy /µV.s : -126.0198 (Exothermic effect) Cooling 0 -1 0.5 Heating -0.5 Peak :1813.08 °C Onset Point :1802.64 °C Enthalpy /µV.s : 127.6076 (Endothermic effect) 1700 1750 1800 Sample temperature/°C DTA traces of HfO2 (99.95% Aldrich) in Ar flow. Heating rate 10 °/min, cooling rate 20 °/min. Baseline correction applied. IMPROVEMENTS: Laser-melted La2O3 sphere, welded tungsten crucible with La2O3 sample, and transparent solidified La2O3 sample after melting in thermal analyzer. The crucibles and samples are positioned on the tungsten sensor plate. Temperature ,°C H X cubic CN6 cubic CN6 C B A 2000 cubic CN6 hex CN7 1600 M. Zinkevich, Progress in Mat Sci 52, 597 (2007) L 2400 mon CN7 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Lanthanum oxide – phase transitions and fusion Reversible DG = DH - T DS DH, DS > 0 for low high Reversible with hysteresis Thermodynamically reversible, but kinetically hindered Thermodynamically irreversible (initial low T phase metastable) Enthalpy of formation convert material chemically to a state with known enthalpy usually a solution) • Thermochemical Cycle • A => solution DH1 • B => same solution DH2 • A => B DH3= DH1 - DH2 • The task is to find a reaction scheme and solvent that lets you do this accurately – – – – A can be elements, B compound A can be binary oxides, B ternary compound A can be end-members, B solid solution or alloy A and B can be polymorphs, materials with different orderidisorder, different surface areas Solution Calorimetry: Solvents • Near room temperature – Water, aqueous acid of base – Hydrofluoric acid – Organic solvents • At high temperature – Molten metals, e.g. Sn – Molten salts, e.g. nitrate or chloride eutectics – Molten oxides High Temperature Oxide Melt Solution Calorimetry • Dissolve oxide samples (5-15 mg) in a molten oxide solvent (20 g) at to form a dilute solution • Difference in heat of solution of reactants and products gives heat of reaction • Oxidative reactions for nitrides, sulfides, selenides, carbides • Needed for ceramic materials which do not dissolve in aqueous solvents Solvents and Systems • Lead borate (2PbO-4B2O3, sodium molybdate (3Na2O-4MoO3), alkali borate • Oxides dissolve • H2O and CO2 evolve as gases • Nitride oxidized to evolved N2 • Sulfide oxidized to dissolved sulfate Background: High-temperatureoxide melt solution calorimetry Custom-built Calvet twin microcalorimeter www.mindat.org drop tube silica glass liner Drop solution setup sample 25 °C alumina plug platinum bubbling tube platinum crucible Inconel block Thermopiles solvent 702 °C Heaters Insulation µV Time Nanovoltmeter Commercial Setaram AlexSYS Calorimeter High-Temperature Calorimetry TTD SOL DS 25oC 25oC 25oC DHDS DHSOL DHTTD 700oC DHTTD 700C 25C 700oC CP dT 700oC DHDS DHTTD DHSOL Thermochemical Cycles Perovskites • 1. AO(xl, 298K) = AO(dissolved, 973K) • 2. BO2(xl, 298K) =BO2(dissolved, 973K) • 3. ABO3(xl, 298K) = ABO3(dissolved, 973K) ______________________________ • 4. AO(xl, 298K) + BO2(xl, 298K) = ABO3(xl, 298K) , H4 = H1 + H2 – H3 • Tolerance factor t = dAO/1.414dBO Gas Adsorption Calorimetry • Combine sensitive microcalorimeter with automated gas dosing system • Measure heat of adsorption and adsorption isotherm simultaneously • Apply to high surface area and microporous materials Volumetric dosing system Calvet-type twin microcalorimeter Micromeritics ASAP2020 Setaram DSC 111 sample thermopiles reference H2O to voltmeter and amplifier Differential (a) and integral (b) heats of H2O adsorption for anatase with surface area of 90, 200 and 240 m2/g and rutile of 61 and 103 m2/g (Levchenko et al. 2006). The Peter A. Rock Thermochemistry Laboratory • A unique suite of equipment and expertise • Can design a calorimetric experiment to suit almost any material and problem High Pressure Phase Transitions • G = E + PV –TS • At a given P,T dG = dE + PdV – TdS • Increasing T, DS has to be positive but DV can have either sign • Increasing P, DV has to be negative but DS can have either sign • Clausius-Clapeyron Equation • (dP/dT)equil = DS/DV Clausius-Clapeyron Equation (dP/dT)equil = DS/DV • For solid-solid reactions, to a good approximation, P-T slope is constant • For melting, the liquid is often more compressible than the solid, so phase boundary is curved, sometimes even showing a maximum • For dehydration or vaporization, strong curvature is seen because gas is much more compressible than condensed phase H2O Phase Diagram Al2SiO5 phase diagram Depth (km) Upper mantle Lower mantle Pressure (GPa) Outer core Inner core Concentric shells of different phase assemblages with sharp discontinuities between them Olivine-spinelloid-spinel at 400 km Spinel- perovskite + periclase at 670 km Core-mantle boundary Phase diagram of Iron 40 (hcp) Pressure, GPa 30 (fcc) 20 10 (bcc) (bcc) liquid 0 0 500 1000 1500 Temperature, K 2000 SILICATE PEROVSKITE IN THE EARTH’S MANTLE • Mantle composition between MgSiO3 and Mg2SiO4 projected on the MgO - SiO2 binary system • Contains a few percent Fe, Al, Ca, other minor elements, +/- H2O • An MgSiO3 - rich perovskite phase predominates at P > 25 GPa (depth > 670 km) Mg2SiO4-Fe2SiO4 phase diagram M2SiO4 transitions olivine – spinelloid - spinel 30 Pressure (GPa) PV 20 IL BP+ST BETA+ST GT PX 10 1000 1500 LIQ 2000 2500 3000 3500 Temperature, K Phase relations in MgSiO3 composition (PX – pyroxene, BETA -wadsleyite, LIQ –liquid, SP –spinel, ST –stishovite, IL – ilmenite, PV -perovskite (After Fei Saxena, Alexandra Navrotsky, 1990)